Doxorubicin prodrug, its preparation method and injectable composition
A technology of doxorubicin and composition, applied in the field of doxorubicin prodrug and its injectable pH-sensitive hydrogel, can solve the problems of drug toxicity damage, increase patient pain, inability to distinguish, etc. The effect of long cleaning time and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
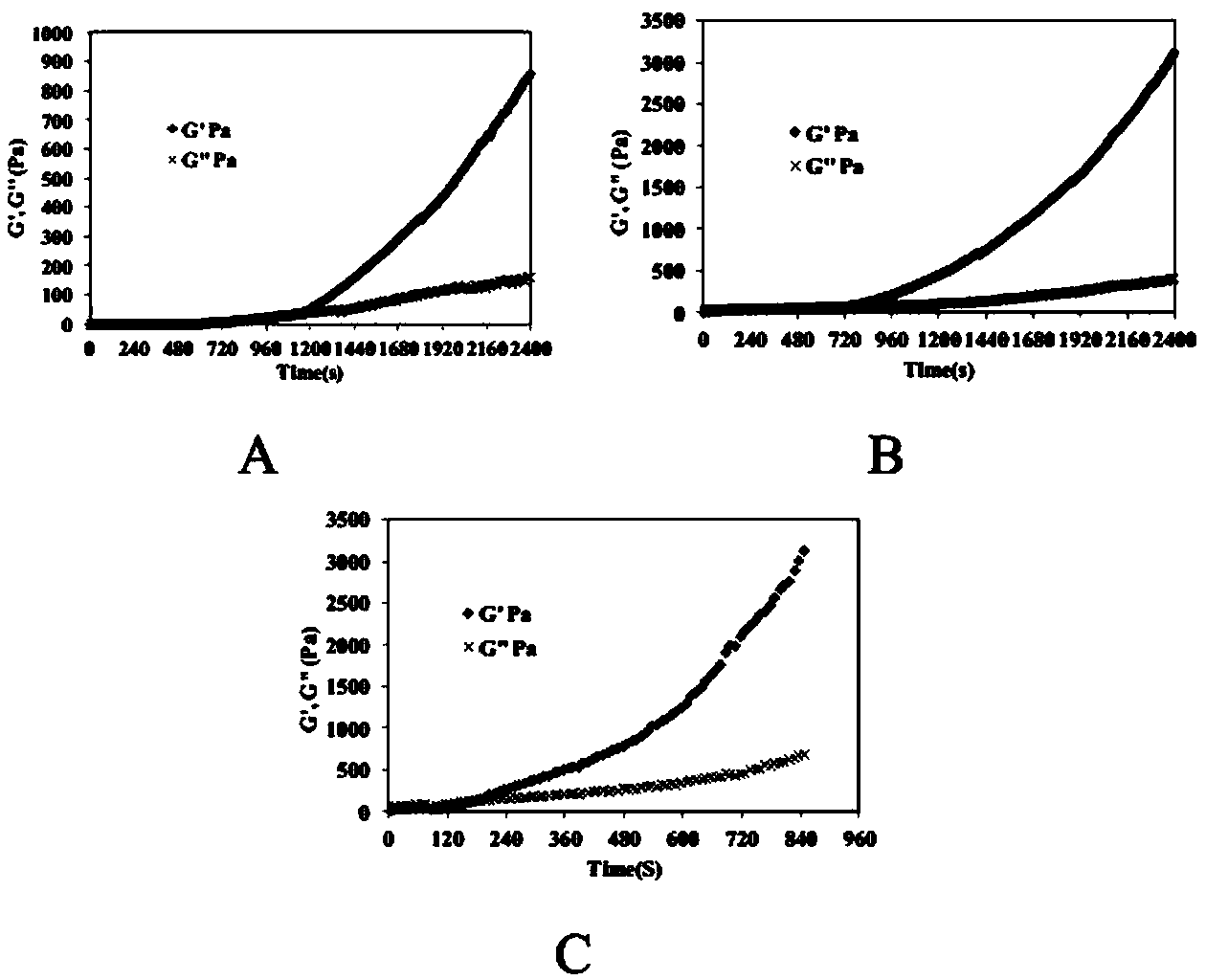
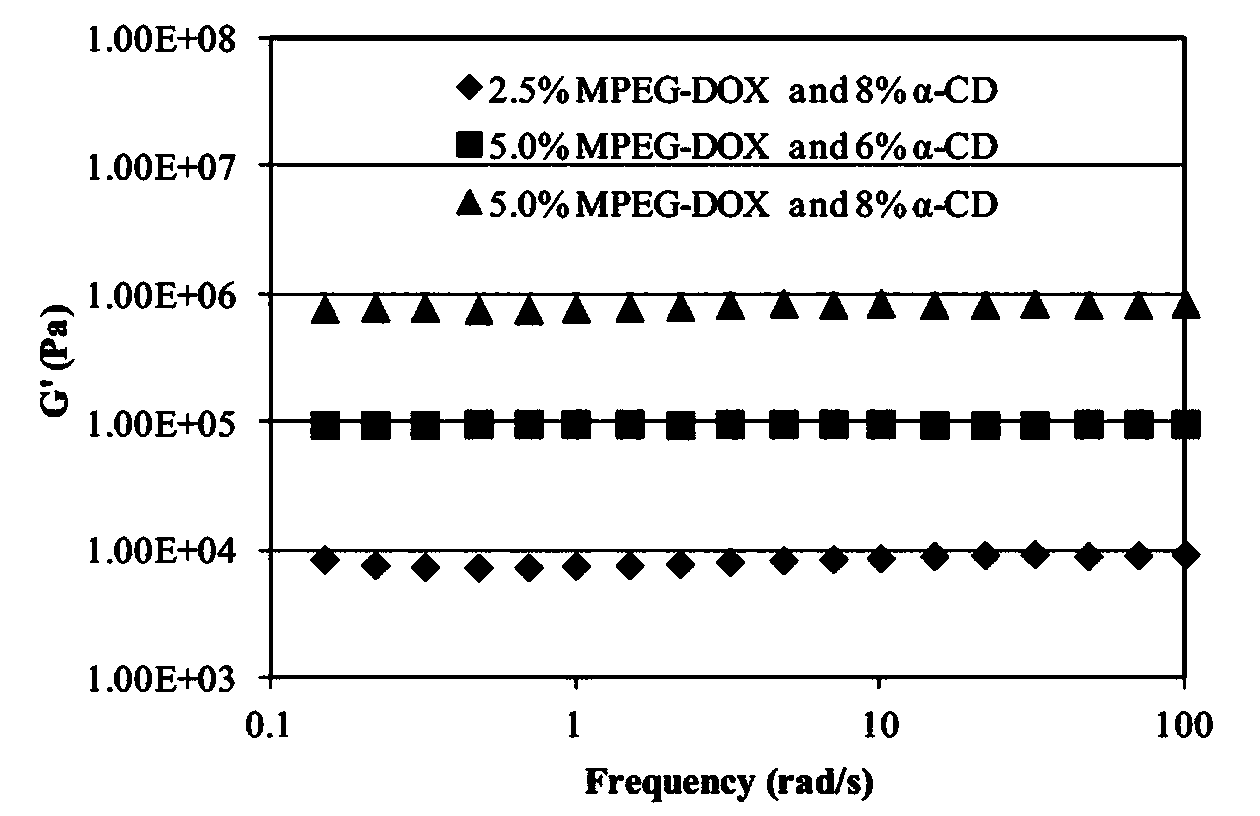
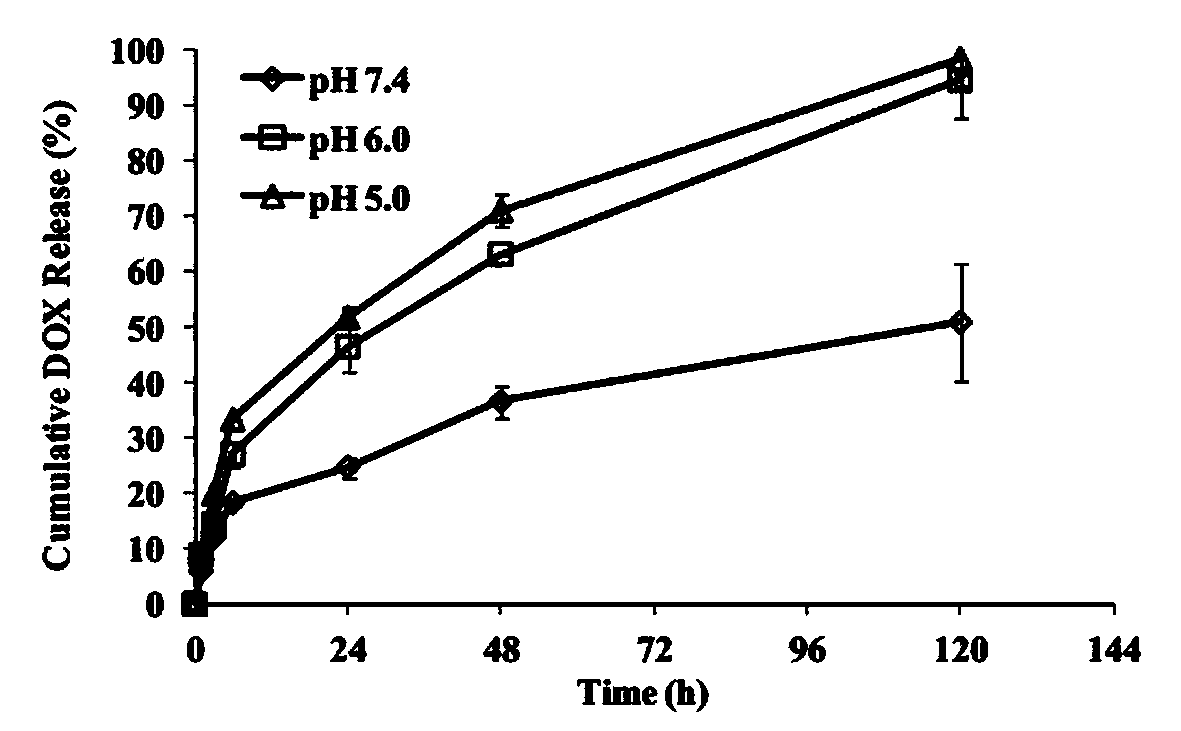
Image
Examples
preparation example Construction
[0046] The preparation method of above-mentioned doxorubicin prodrug, comprises the steps:
[0047] Dissolve MPEG-hydrazide and doxorubicin in an anhydrous polar solvent, stir to make the reaction complete, add excess triethylamine to continue the reaction, and purify to obtain the doxorubicin prodrug, which is named MPEG-DOX.
[0048] As a further improvement of the present invention, the synthetic method of MPEG-hydrazide comprises the steps:
[0049] 1) Take excess hydrazine sulfate salt and dissolve it in water, adjust its pH to be alkaline, and obtain reaction solution A;
[0050] 2) Add methoxypolyethylene glycol succinimidyl acetate into the reaction solution A, stir to complete the reaction, dialyze to remove unreacted small molecules, and freeze-dry to obtain purified MPEG-hydrazide.
[0051] As a further improvement of the present invention, add NaOH solution to adjust the pH of the hydrazine sulfate salt solution to 8.5 to 10. The molecular weight of the hydrazine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com