Lithium ion battery Li3MnO4 positive material doped with vanadium and preparation method thereof
A technology of lithium ion battery and positive electrode material, which is applied to battery electrodes, circuits, electrical components, etc., to achieve the effect of improving discharge performance and reducing impedance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
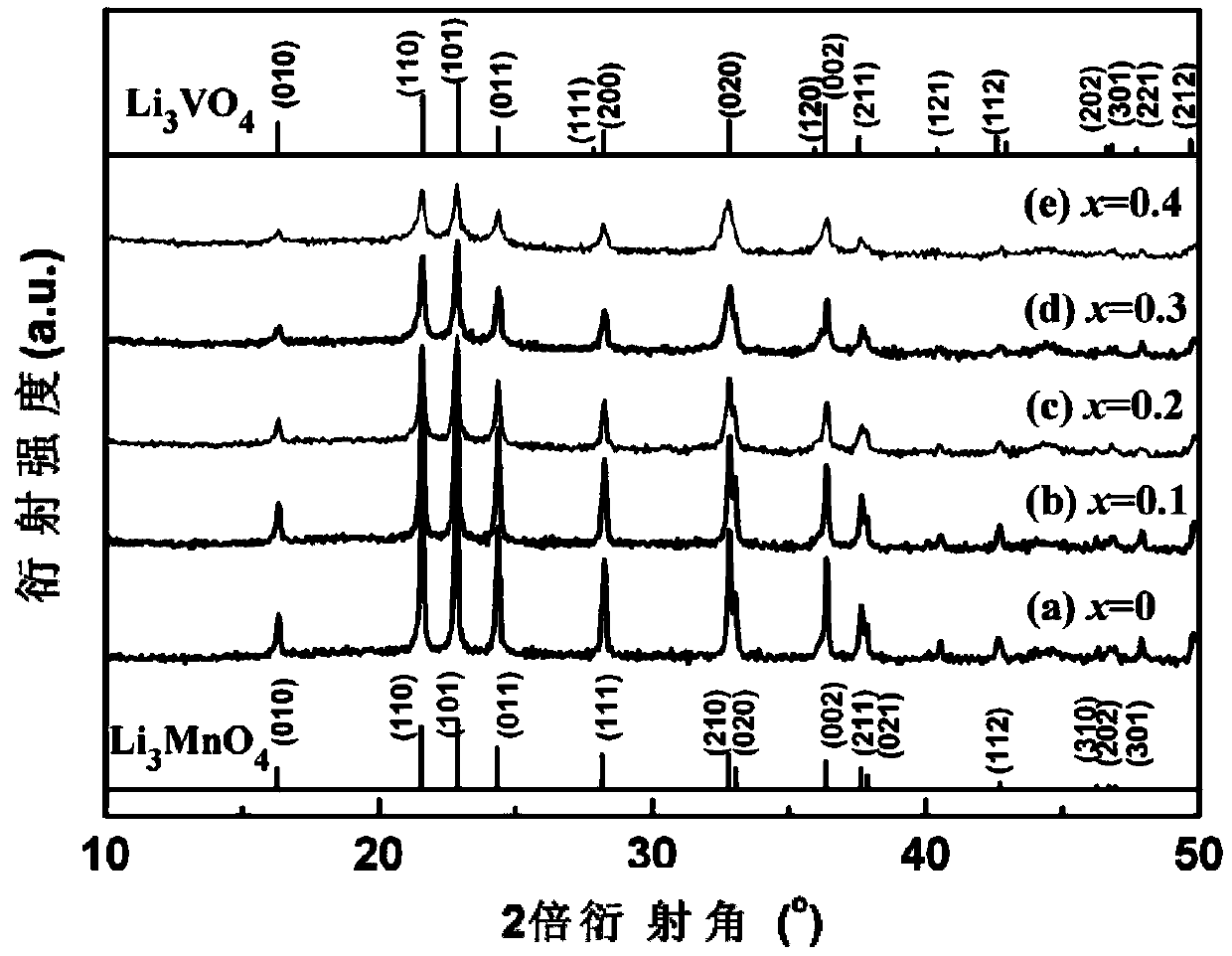
[0027] Example 1: Li 3 Mn 0.9 V 0.1 O 4 Preparation
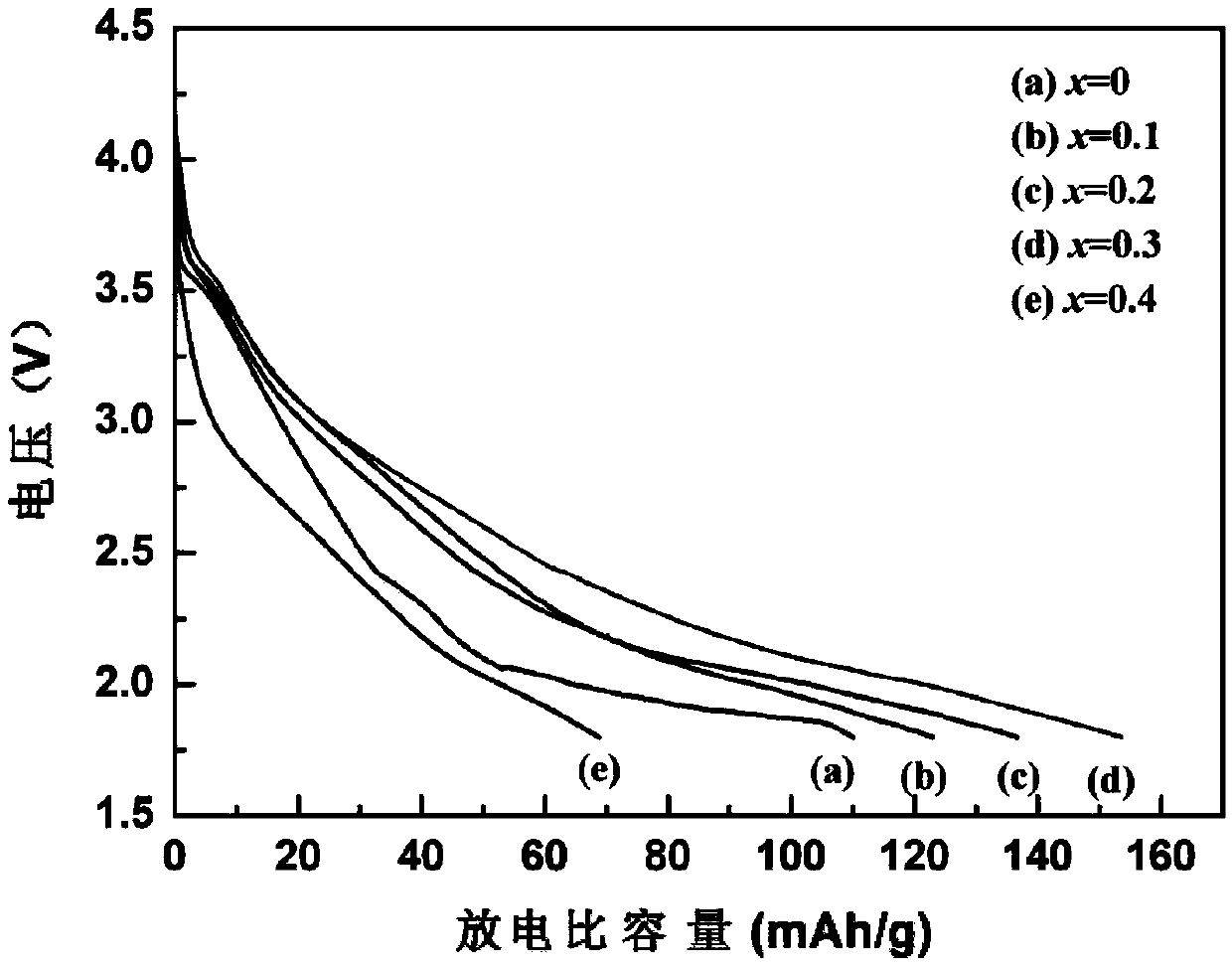
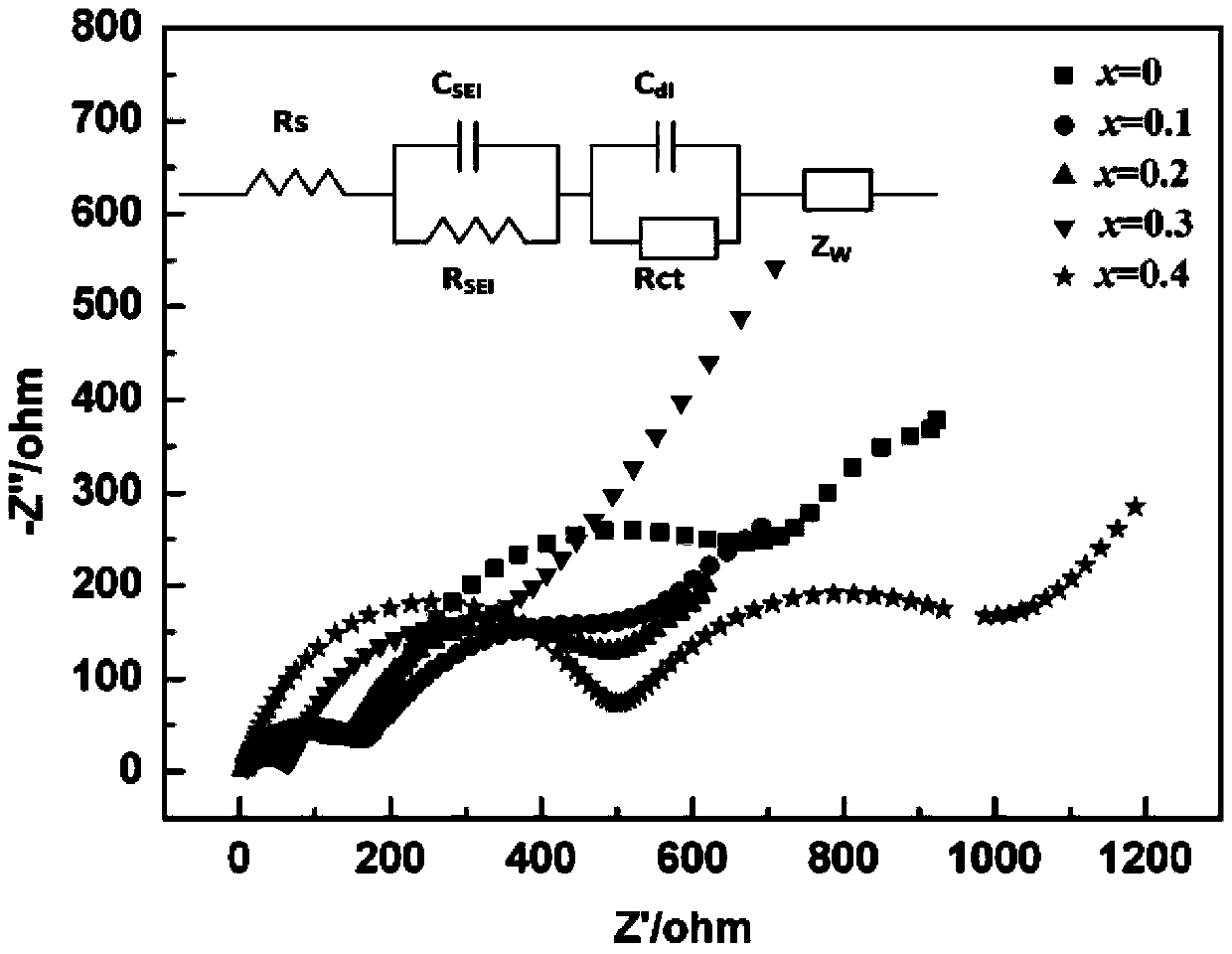
[0028] KMnO 4 The solution is converted to LiMnO through lithium cation exchange resin 4 Solution, then LiMnO 4 LiMnO is obtained after the solution is vacuum dried at 50℃ for 24h 4 ·3H 2 O powder. Weigh LiOH·H with a molar ratio of 2.1:0.9:0.1 2 O, LiMnO 4 ·3H 2 O and NH 4 VO 3 Mix and grind, calcine in a tube furnace with oxygen atmosphere, grind at 70~120℃ every 10℃, grind at 125℃, keep for 1 hour, then heat to 170℃ and keep for 2.5h to synthesize Li 3 Mn 0.9 V 0.1 O 4 Cathode material. Using the synthetic material as the positive electrode and the lithium sheet as the negative electrode, electrochemical tests and charge-discharge tests were carried out. Its discharge capacity is 123mAh / g at a current density of 7mA / g, which is an increase of 13mAh / g compared to the undoped material.
Embodiment 2
[0029] Example 2: Li 3 Mn 0.8 V 0.2 O 4 Preparation
[0030] KMnO 4 The solution is converted to LiMnO through lithium cation exchange resin 4 Solution, then LiMnO 4 LiMnO is obtained after the solution is vacuum dried at 50℃ for 24h 4 ·3H 2 O powder. Weigh LiOH·H in a molar ratio of 2.2:0.8:0.2 2 O, LiMnO 4 ·3H 2 O and NH 4 VO 3 Mix and grind, calcine in a tube furnace with oxygen atmosphere, grind at 70~120℃ every 10℃, grind at 125℃, keep for 1 hour, then heat to 170℃ and keep for 2.5h to synthesize Li 3 Mn 0.8 V 0.2 O 4 Cathode material. Using the synthetic material as the positive electrode and the lithium sheet as the negative electrode, electrochemical tests and charge-discharge tests were carried out. Its discharge capacity is 137mAh / g at a current density of 7mA / g, which is an increase of 27mAh / g over the undoped material.
Embodiment 3
[0031] Example 3: Li 3 Mn 0.7 V 0.3 O 4 Preparation
[0032] KMnO 4 The solution is converted to LiMnO through lithium cation exchange resin 4 Solution, then LiMnO 4 LiMnO is obtained after the solution is vacuum dried at 50℃ for 24h 4 ·3H 2 O crystal powder. Weigh LiOH·H in a molar ratio of 2.3:0.7:0.3 2 O, LiMnO 4 ·3H 2 O and NH 4 VO 3 Mix and grind, calcine in a tube furnace with oxygen atmosphere, grind at 70~120℃ every 10℃, grind at 125℃, keep for 1 hour, then heat to 170℃ and keep for 2.5h to synthesize Li 3 Mn 0.7 V 0.3 O 4 Cathode material. Using the synthetic material as the positive electrode and the lithium sheet as the negative electrode, electrochemical tests and charge-discharge tests were carried out. Its discharge capacity is 153mAh / g at a current density of 7mA / g, which is 43mAh / g higher than that of the undoped material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com