Preparation method of prednisolone acetate
A technology of prednisolone acetate and acidic conditions is applied in the field of preparation of steroid compounds, which can solve the problems of complicated operation, easy decomposition, unstable intermediates and the like, and achieves simple separation and purification operation, high material utilization rate, The effect of low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
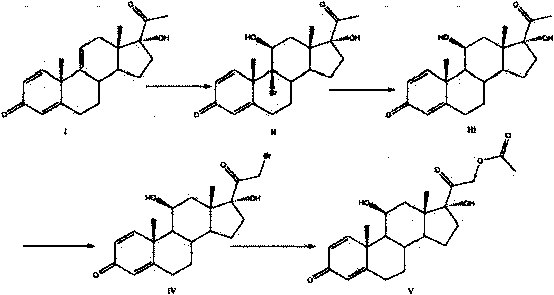
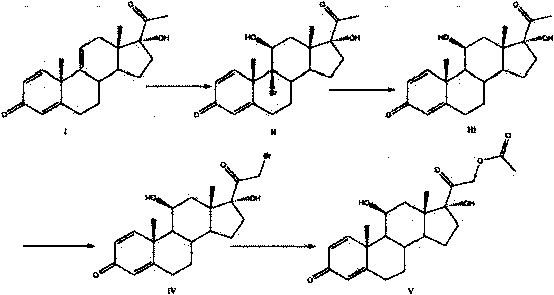
Image
Examples
Embodiment 1
[0033] (1) Add 10g of raw material (I), 50ml of acetone, and 20ml of glacial acetic acid into the reactor, stir, cool down to 0°C, add 30ml of acetone dissolved with 9g of NBS within 30 minutes, and react at 5-10°C for 2 hours. Acetone was distilled off.
[0034] (2) Add 30-50ml of ether into the reactor, slowly add 3.8g-4.2g of zinc powder, and carry out the reduction at room temperature for 2h. Stirring was continued for 15 minutes after the end of the TLC detection reaction.
[0035] (3) Add 0.1g catalyst AlCl to the reactor 3 , Stir at -5~0°C, slowly add 30ml of diethyl ether solution containing 5.5g of bromine within 30 minutes; keep the reaction temperature constant, and react for 2h.
[0036] (4) Ether is evaporated and recovered, and the acetone evaporated in step (1) is added as a solvent; 24g of sodium hydroxide powder is added to the reactor, and then 3ml of triethylamine is added, heated to 50-55°C under stirring for reaction 3 Hours, TLC detects the end of the ...
Embodiment 2
[0039] Also using 10 g of the compound of formula I as a raw material, the method in Example 1 was used to carry out steps (1) to (3);
[0040] (1) Add raw material (I) 10g and 50ml acetone, 20ml glacial acetic acid to acid-resistant reactor 1, stir, cool down to 0°C, add 30ml of acetone dissolved with 9g NBS within 30 minutes, and react at 5-10°C 2 hours. Acetone was distilled off.
[0041] (2) Add 30-50ml ether to reactor 1, slowly add zinc powder, and carry out reduction at room temperature for 2 hours. Stirring was continued for 15 minutes after the TLC reaction was completed.
[0042] (3) Add 0.2g catalyst AlCl to reactor 1 3 , Stir at -5~0°C, slowly add 30ml of diethyl ether solution containing 5.5g of bromine within 30 minutes; keep the reaction temperature constant, and react for 2h.
[0043] (4) Add sodium hydroxide to the reactor 1 to adjust the pH to neutral, then the zinc salt will precipitate out, filter the solution to remove the insoluble matter, wash the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com