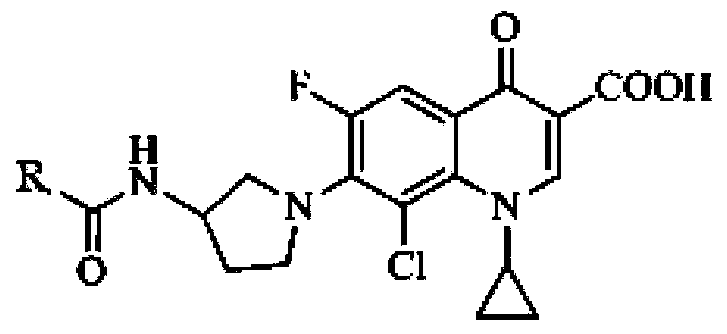
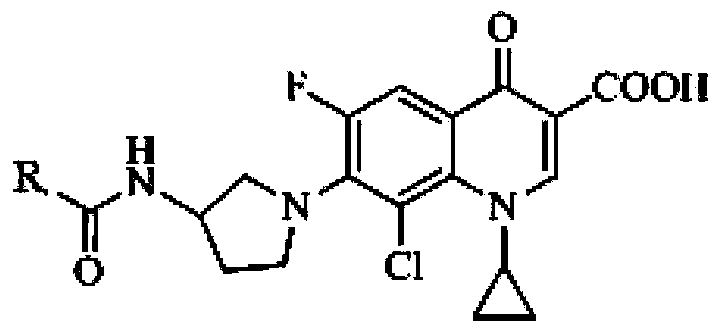
Application of clinafloxacin amino derivatives and medicinal salts thereof in preparing antitubercular medicaments
A technology of derivatives and star amino, which is applied to compounds in the fields of pharmacy and chemistry, and can solve unclear problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
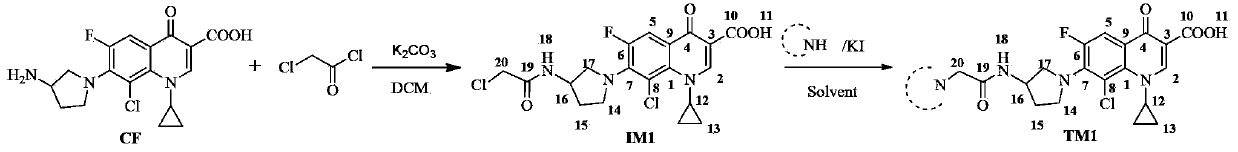
[0018] Example 1. Synthesis of compound TM1
[0019]
[0020] Add clinfloxacin (CF), K to the reaction flask 2 CO 3 And an appropriate amount of dichloromethane (DCM), chloroacetyl chloride in DCM solution, CF, K 2 CO 3 The molar ratio of chloroacetyl chloride to chloroacetyl chloride is 10:25:15-25, after dropping, the reaction is stirred under ice bath, and the reaction progress is monitored by TLC (Thin Layer Chromatography). After the reaction was completed, a yellow-green turbid liquid was obtained, which was filtered off with suction, the filter cake was washed with DCM, the washing liquid was combined with the filtrate, and rotary evaporated to dryness to obtain intermediate IM1.
[0021] Add the amine component and K to the reaction flask 2 CO 3 And a catalytic amount of KI and an appropriate amount of solvent. After stirring for 30 minutes at room temperature, add intermediates IM1, IM1 and K 2 CO 3 The molar ratio of the feed to the amine component is 1:3:2, the temperatur...
Embodiment 2
[0035] Example 2. Synthesis of compound TM2
[0036]
[0037] Add CF and appropriate amount of chloroform to the reaction flask. After stirring evenly, slowly add the chloroform solution of bis(trichloromethyl) carbonate (BTC) dropwise. After the dropwise addition is completed, stir vigorously for 7 hours under ice bath and then add dropwise slowly. The molar ratio of triethylamine (TEA), CF, BTC and TEA is 17:6:10. After the dropping is completed, the reaction is carried out at room temperature, and the reaction progress is monitored by TLC. After the reaction was completed, the filter cake was washed with DCM, the washing liquid was combined with the filtrate, water was added, and the pH was adjusted to 4 to 5 with 2N HCl, and then extracted with DCM twice. The organic phase was collected and washed with saturated brine twice. Drying with sodium sulfate and filtering, the filtrate is concentrated by rotary evaporation, and purified by flash column chromatography to obtain inter...
Embodiment 3
[0048] Example 3. Synthesis of compound TM3
[0049]
[0050] Add 12mmol of the amino acid Boc-AA-OH whose amino group is protected by tert-butoxycarbonyl (Boc) and 20mL of DCM into the reaction flask, stir for 1min, add 12mmol of 1-hydroxybenzotriazole (HOBt) and bicyclic ring under ice bath 15mmol of hexylcarbodiimide (DCC), stir for 1 min, then add 1 mL of TEA dropwise, stir under ice bath for about 30 min, until all the raw materials have formed activated ester, suction filtration, the filter cake is washed with DCM, the washing liquid and the filtrate are combined and added Clinfloxacin was 10 mmol, and the reaction was stirred at room temperature, and the progress of the reaction was monitored by TLC. After the reaction is complete, filter with suction, wash the filter cake with DCM, combine the washing liquid and the filtrate, and use 10% (w / w) citric acid solution and 0.5mol / L NaHCO respectively 3 Wash solution, saturated NaCl solution, collect organic phase, anhydrous Na...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com