C3 polypeptide supramolecular helical polymer and preparation method thereof
A supramolecular and polymer technology, applied in the field of C3 polypeptide supramolecular helical polymer and its preparation, can solve the problems of expensive raw materials and lack of functionalization of molecules, and achieve the effects of simple preparation, reliable chirality and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
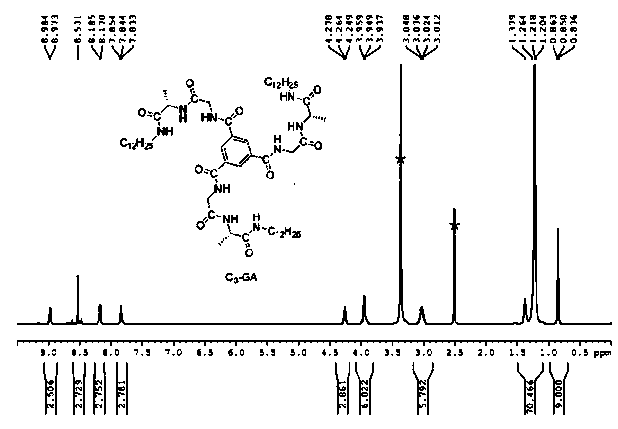
[0041] Take 2.50 mg of C 3 -GA monomer was added into 10 mL of tetrahydrofuran, and then ultrasonic and heated repeatedly until the monomer was completely dissolved. The solution was then left for 12 hours.
Embodiment 2
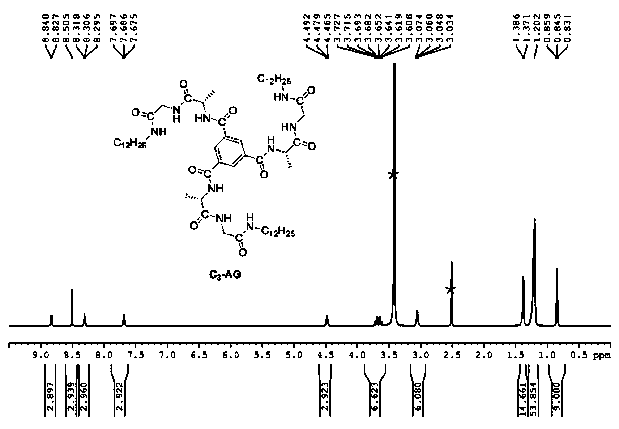
[0043] take multiple copies of C 3 -AG monomer, 2.5 mg each, was added to 10 mL of methanol, 10 mL of propanol, 10 mL of isopropanol, 10 mL of n-butanol, 10 mL of sec-butanol and 10 mL of tetrahydrofuran, and then Repeated sonication and heating until the monomer is completely dissolved. The solution was then left for 12 hours.
Embodiment 3
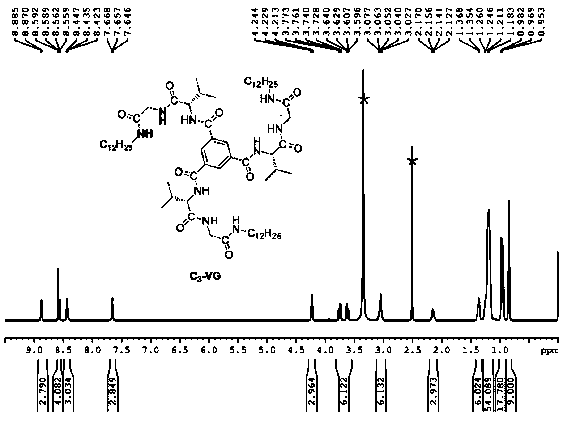
[0045] Take 2.50 mg of C 3 -VG monomer was added into 10 mL of tetrahydrofuran, and then ultrasonic and heated repeatedly until the monomer was completely dissolved. The solution was then left for 12 hours.
[0046] Performance Tests and Uses
[0047] The circular dichroism of the above solutions was measured by circular dichroism (CD). Through the CD spectrum ( Figure 4 ) It can be seen that C 3 The tetrahydrofuran solution of -GA monomer showed negative and positive peaks at 243 nm and 215 nm, respectively, confirming that C 3 - The supramolecular helical polymer formed by layer-by-layer self-assembly of GA monomers has a right-handed helix, and the atomic force microscope (see Figure 7 ), observed that C 3 -GA assemblies are multi-strand winding fibers with helices.
[0048] C 3 THF solution of -AG monomer ( Figure 4 ) have negative, positive and negative peaks at 260 nm, 245 nm and 212 nm, respectively, proving that the supramolecular polymer has a left-hande...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com