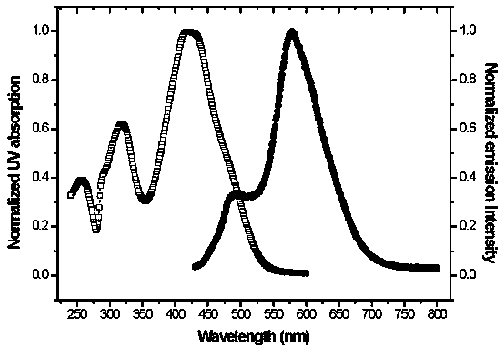
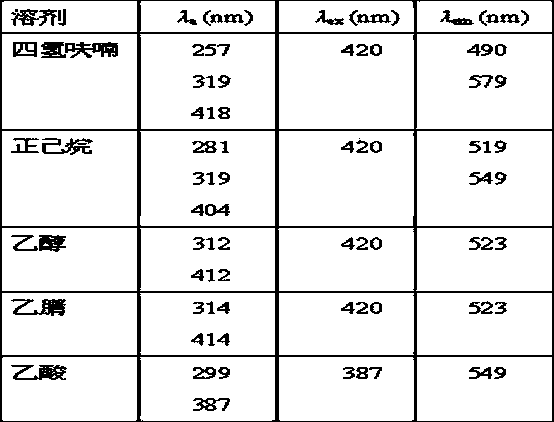
Bifluorescence-emission organic light-emitting material and preparation method thereof
A luminescent material and dual fluorescence technology, which is applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problem of limited emission wavelength region, and achieve the effects of enhanced electron pulling, easy storage, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
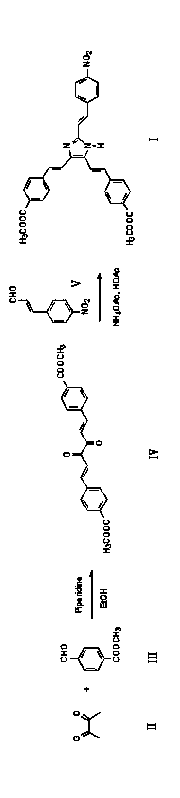
[0025] 4,4'-(2,2'-(2-(4-nitrostyryl)-1H-imidazol-4,5-diyl))bis(ethylene-2,1-diyl)dibenzoic acid Preparation of Dimethyl Ester (Compound I)
[0026] (1) Synthesis of dimethyl 4,4'-(3,4-dioxo-1,5-hexadiene-1,6-diyl)dibenzoate (compound Ⅳ)
[0027] In a dry 100 ml round bottom flask, add 2,3-butanedione (compound Ⅱ) (0.01 mol), methyl 4-formylbenzoate (compound Ⅲ) (0.02 mol), and 35 ml of absolute ethanol , and then 0.5 ml of piperidine was added to the solution under rapid stirring, and then stirred and refluxed for 7 hours. After cooling to room temperature, a yellow solid precipitated, which was filtered under reduced pressure, washed twice with absolute ethanol, recrystallized with a mixed solvent of ethanol-N,N-dimethylformamide, and dried in vacuo to obtain orange crystals with a yield of 19 %. The melting point is 249-251 ℃
[0028] (2) 4,4'-(2,2'-(2-(4-nitrostyryl)-1H-imidazole-4,5-diyl))bis(ethylene-2,1-diyl) Synthesis of Dimethyl Dibenzoate (Compound I)
[0029] I...
Embodiment 2
[0033] 4,4'-(2,2'-(2-(4-nitrostyryl)-1H-imidazol-4,5-diyl))bis(ethylene-2,1-diyl)dibenzoic acid Preparation of Dimethyl Ester (Compound I)
[0034] According to the method of Example 1, obtained by two-step reaction, the difference is that in step 1, the mol ratio of 2,3-butanedione (compound II) and 4-formyl formic acid methyl ester (compound III) is 1: 2.1, yield rate is 20%; In step 2, 4,4'-(3,4-dioxo-1,5-hexadiene-1,6-diyl)dibenzoic acid dimethyl ester (compound IV ), 4-nitrocinnamaldehyde (compound V) and ammonium acetate in a molar ratio of 1: 1.1: 15, and the yield was 63%.
[0035]
Embodiment 3
[0037] 4,4'-(2,2'-(2-(4-nitrostyryl)-1H-imidazol-4,5-diyl))bis(ethylene-2,1-diyl)dibenzoic acid Preparation of Dimethyl Ester (Compound I)
[0038] According to the method of Example 1, it is obtained by two-step reaction, and the difference is that step 2 is carried out according to the following microwave radiation method.
[0039]Add dimethyl 4,4'-(3,4-dioxo-1,5-hexadiene-1,6-diyl)dibenzoate (compound IV) into a dry 100 ml Erlenmeyer flask ( 0.001mol), 4-nitrocinnamaldehyde (compound Ⅴ) (0.001mol) and ammonium acetate (0.015mol), and 25 mL of glacial acetic acid, stir well, put in microwave oven, medium-low fire radiation reaction, take out and stir for more times, the reaction time was 12 minutes. After the reaction was completed, cool to room temperature, pour the reaction solution into ice water under rapid stirring, adjust the pH to 7 with 10% aqueous sodium hydroxide solution, filter the obtained solid under reduced pressure, wash with water several times, and dry at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com