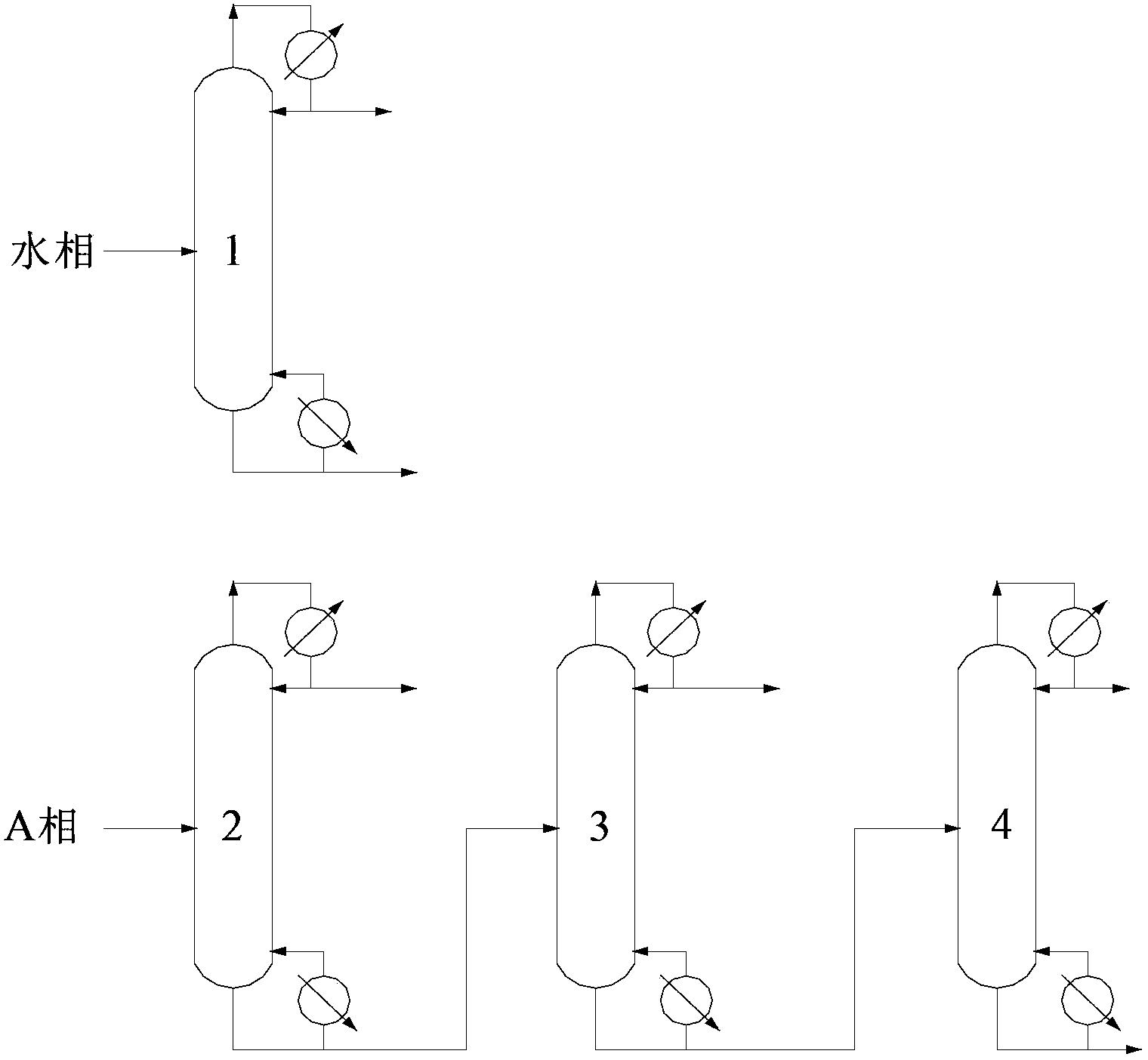Separation method for epichlorohydrin
A technology of epichlorohydrin and separation method, which is applied in the field of epichlorohydrin separation, can solve the problems of difficult phase separation, high energy consumption of distillation separation, etc., achieve short phase separation time, reduce requirements and difficulty and ease of extraction operation Achieved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
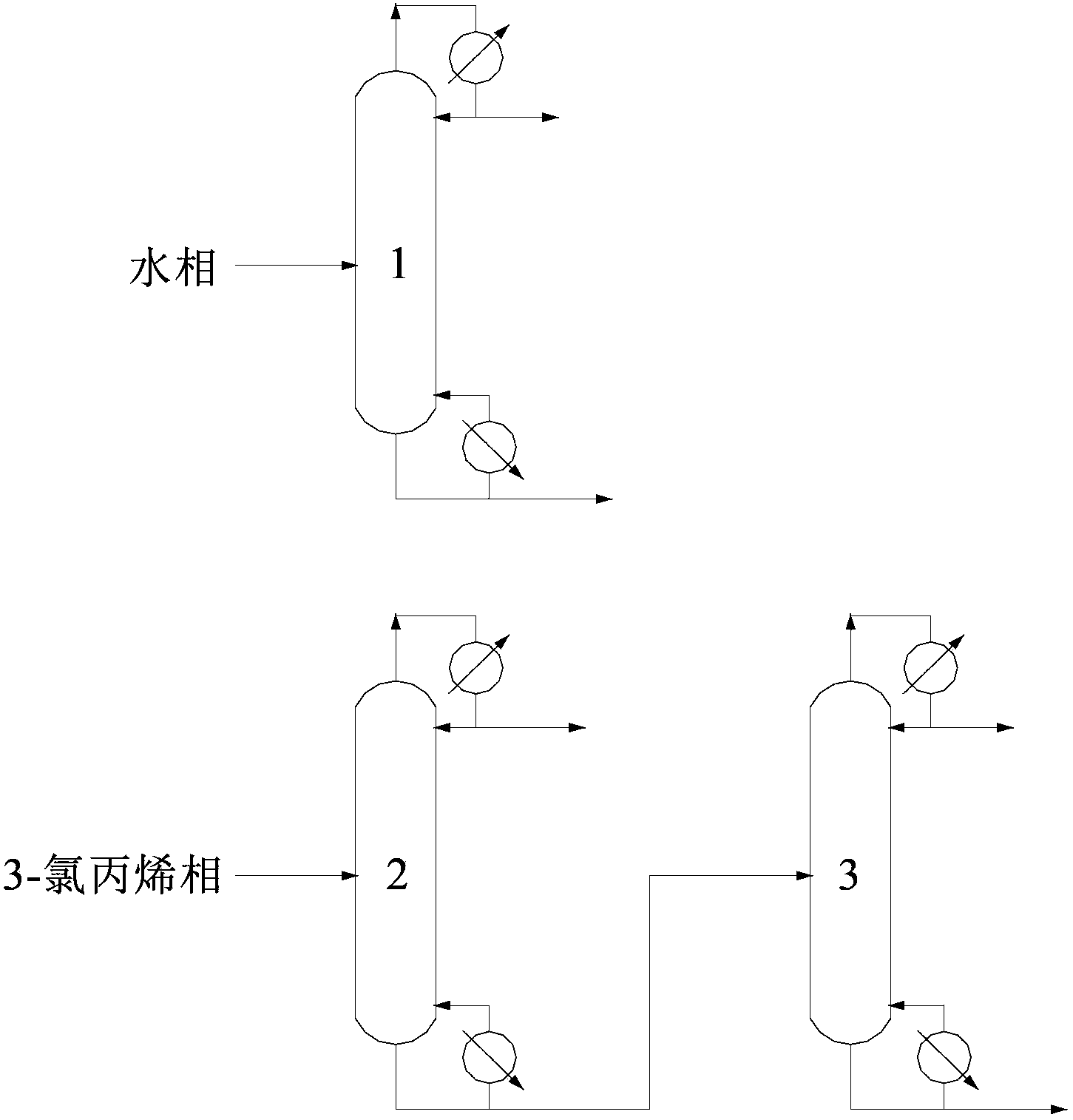
[0069] This embodiment is used to illustrate the separation method of epichlorohydrin provided by the present invention.
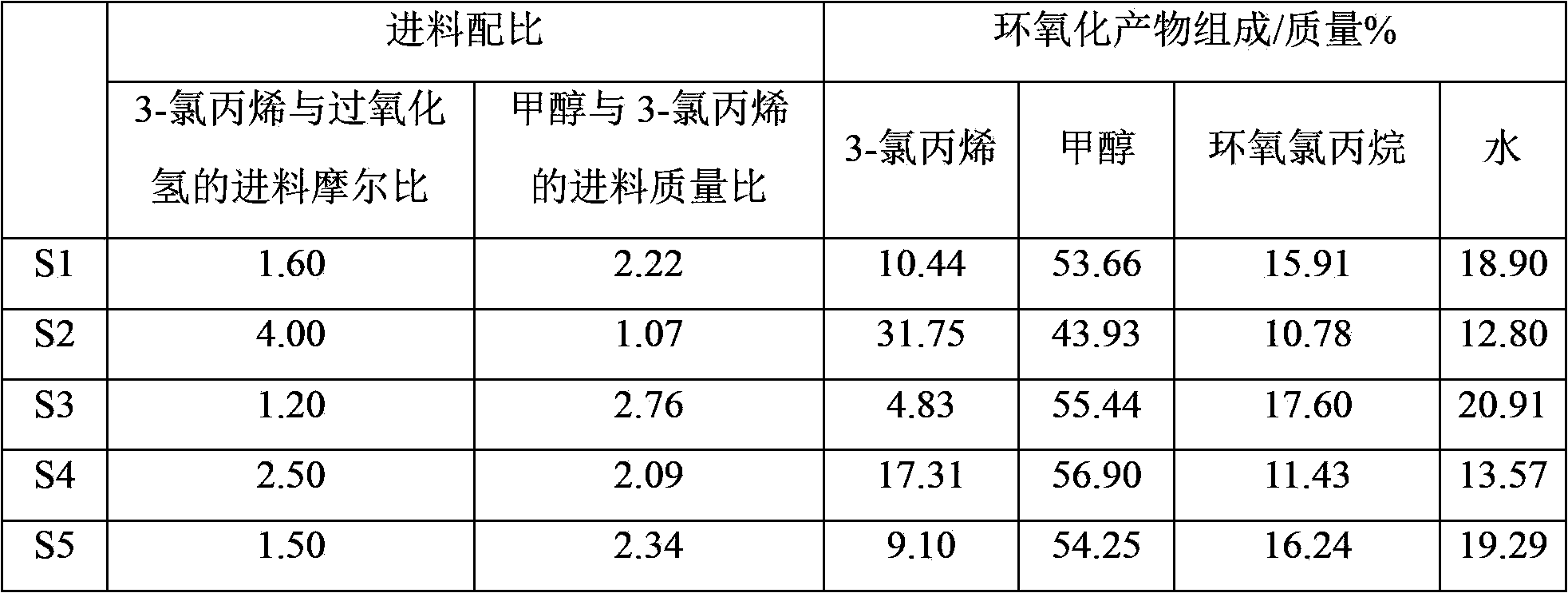
[0070] At 28°C, 100 parts by mass of the known solution S1 containing epichlorohydrin, methanol, 3-chloropropene and water was mixed with 49.9 parts by mass of water (the density at 20°C was 998.2 kg / m3, The boiling point is 100°C) contacting and mixing, after standing to separate layers, collect the heavy phase of the lower layer, and then use 70.0 parts by mass of ethyl trichloroacetate (TCH (Shanghai) Chemical Industry Development Co., Ltd., the density at 20°C is 1383.6 kg / m3, the boiling point is 168°C) and 34.8 parts by mass of 3-chloropropene (industrial chloropropene, Baling Petrochemical Co., Ltd., the content of 3-chloropropene is 98 mass%, and the density at 20°C is 939.2 kg / m3, with a boiling point of 45°C), the mixed organic solvent is divided into five parts of equal mass and extracted five times to extract the upper light phase, and all the ...
Embodiment 2
[0084] This embodiment is used to illustrate the separation method of epichlorohydrin provided by the present invention.
[0085] At 15°C, 100 parts by mass of the known solution S2 containing epichlorohydrin, methanol, 3-chloropropene and water is first mixed with 50.1 parts by mass of water (the density at 20°C is 998.2 kg / m3, The boiling point is 100°C), contact and mix, after standing to separate layers, collect the heavy phase of the lower layer, and then use 40.0 parts by mass of ethyl trichloroacetate (TSI (Shanghai) Chemical Industry Development Co., Ltd., the density at 20°C is 1383.6 kg / m3, the boiling point is 168°C) and 49.9 parts by mass of 3-chloropropene (industrial chloropropene, Baling Petrochemical Co., Ltd., the content of 3-chloropropene is 98 mass%, and the density at 20°C is 939.2 kg / m3, with a boiling point of 45°C), the mixed organic solvent is divided into five parts of equal mass and extracted five times to extract the upper light phase, and all the h...
Embodiment 3
[0097] This embodiment is used to illustrate the separation method of epichlorohydrin provided by the present invention.
[0098] At 28°C, 100 parts by mass of the known solution S3 containing epichlorohydrin, methanol, 3-chloropropene and water was first mixed with 60.2 parts by mass of water (the density at 20°C was 998.2 kg / m3, The boiling point is 100°C) contacting and mixing, after standing to separate layers, collect the heavy phase of the lower layer, and then use 50.0 parts by mass of p-chloroanisole (TSI (Shanghai) Chemical Industry Development Co., Ltd., the density at 20°C is 1160 kg / m3, the boiling point is 200°C) and 49.9 parts by mass of 3-chloropropene (industrial 3-chloropropene, Baling Petrochemical Co., Ltd., the content of 3-chloropropene is 98% by mass, at 20°C Density is 939.2 kg / m3, boiling point is 45 ℃) the mixed organic solvent is divided into five parts of equal mass and extracted five times to extract the upper light phase, and combine all the heavy ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com