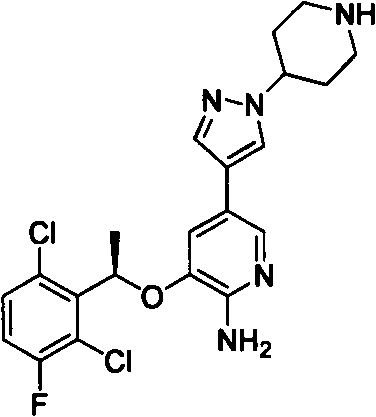
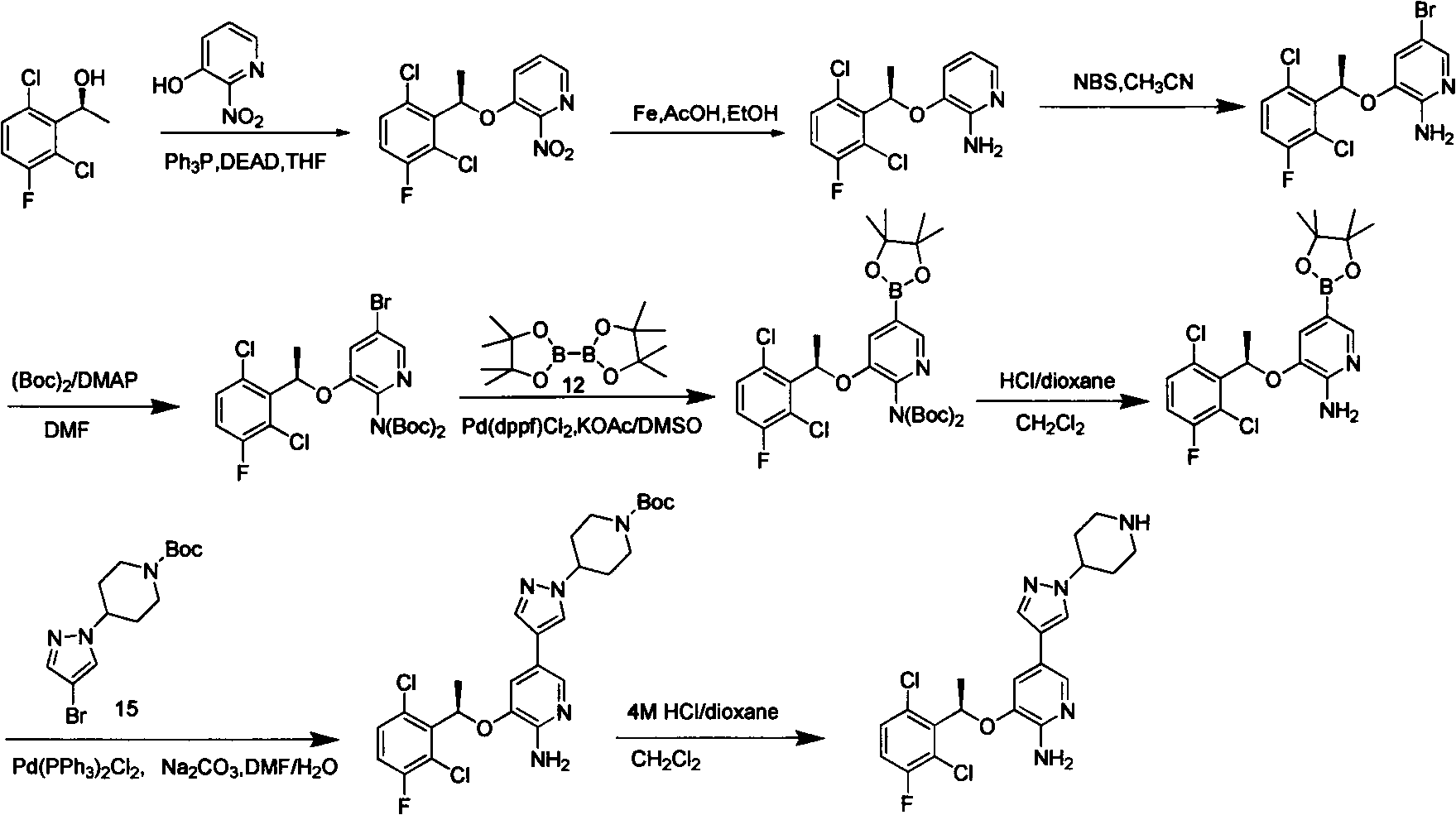
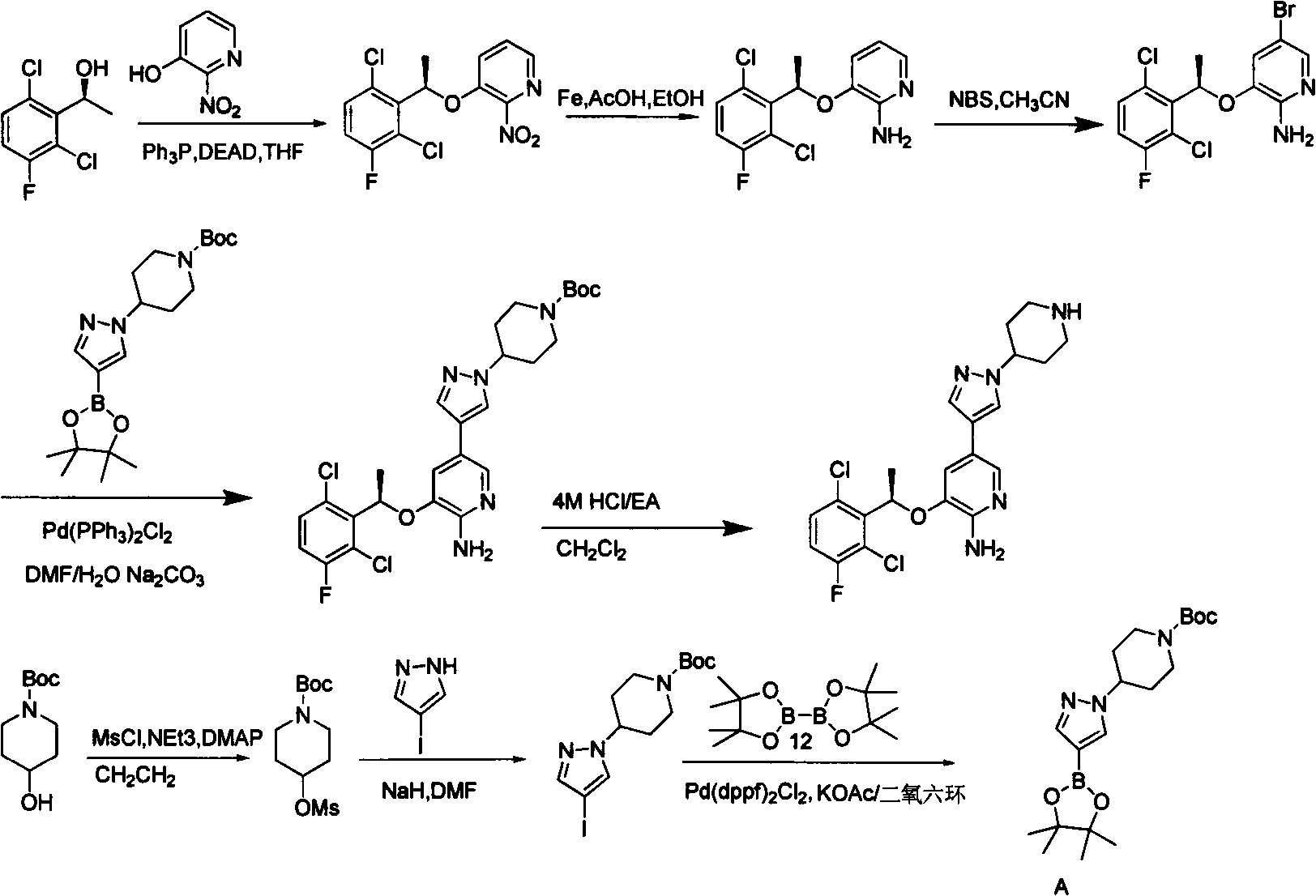
Pyridine derivatives and preparation methods thereof
A derivative, pyridine technology, applied in the field of pyridine derivatives and its preparation, can solve the problems of many by-products, high cost, cumbersome operation, etc., and achieve the effect of less by-products, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Tert-butyl-4-(4-(6-(bis(tert-butoxycarbonyl)amino)-5-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridine Preparation of -3-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylate (1)
[0037]Add compound 2 (racemate) (2.10g, 3.35mmol) in 20mlDMF, then add compound 3 (1.33g, 4.02mmol), sodium carbonate aqueous solution (3.0 molar equivalent of compound 2), nitrogen replacement 5 times, add 0.13gPd(PPh 3 ) 3 Cl 2 , replaced with nitrogen 5 times, heated to 80-90°C, then reacted and stirred for 8h, cooled the reaction to room temperature, diluted with ethyl acetate, filtered with diatomaceous earth, washed with ethyl acetate, and the filtrate was washed with water and saturated brine in turn, anhydrous It was dried over sodium sulfate, concentrated, and 1.5 g of light yellow solid was obtained by column chromatography (petroleum ether / ethyl acetate=0-100% gradient elution). Yield: 60%. ee=0%. m / z (MH + )750, 1 H-NMR (400MHz, CDCl3) δ1.27-1.43 (S, 18H), δ1.47-1.55S, 9H) ...
Embodiment 2
[0038] Example 2 (tert-butyl-4-(4-(6-(bis(tert-butoxycarbonyl)amino)-5-(1R-(2,6-dichloro-3-fluorophenyl)ethoxy) Pyridin-3-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylate)
[0039] Add compound 2 (2.10g, 3.35mmol) of R type in 20mlDMF, then add compound 3 (1.33g, 4.02mmol), sodium carbonate aqueous solution (3.0 molar equivalent of compound 2), nitrogen replacement 5 times, add 0.13gPd (PPh 3 ) 3 Cl 2 , replaced with nitrogen 5 times, heated to 80-90°C, then reacted and stirred for 8h, cooled the reaction to room temperature, diluted with ethyl acetate, filtered with diatomaceous earth, washed with ethyl acetate, and the filtrate was washed with water and saturated brine in turn, anhydrous It was dried over sodium sulfate, concentrated, and subjected to flash column chromatography (petroleum ether / ethyl acetate=0-100% gradient elution) to obtain 1.6 g of light yellow solid. Yield: 64%. ee = 99.4%. m / z (MH + )750, 1 H-NMR (400MHz, CDCl3) δ1.27-1.43 (S, 18H), δ1.47-1.55S, 9H...
Embodiment 3
[0040] Example 3 (tert-butyl-4-(4-(6-(bis(tert-butoxycarbonyl)amino)-5-(1S-(2,6-dichloro-3-fluorophenyl)ethoxy) Pyridin-3-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylate)
[0041] Add compound 2 (2.10g, 3.35mmol) of S type in 20mlDMF, then add compound 3 (1.33g, 4.02mmol)), sodium carbonate aqueous solution (3.0 molar equivalent of compound 2), nitrogen replacement 5 times, add 0.13 gPd(PPh 3 ) 3 Cl 2 , replaced with nitrogen 5 times, heated to 80-90°C, then reacted and stirred for 8h, cooled the reaction to room temperature, diluted with ethyl acetate, filtered with diatomaceous earth, washed with ethyl acetate, and the filtrate was washed with water and saturated brine in turn, anhydrous It was dried over sodium sulfate, concentrated, and subjected to flash column chromatography (petroleum ether / ethyl acetate=0-100% gradient elution) to obtain 1.6 g of light yellow solid. Yield: 64%. ee > 99%. m / z (MH + )750, 1 H-NMR (400MHz, CDCl3) δ1.27-1.43 (S, 18H), δ1.47-1.55S, 9H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com