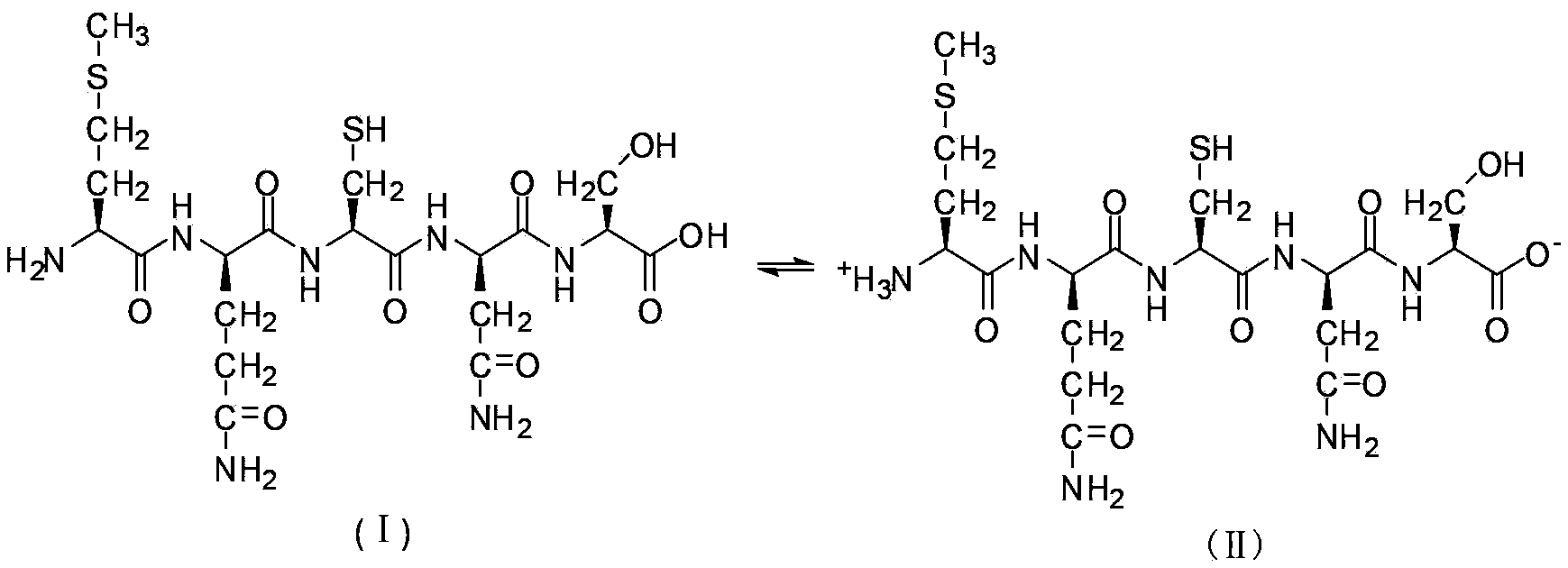
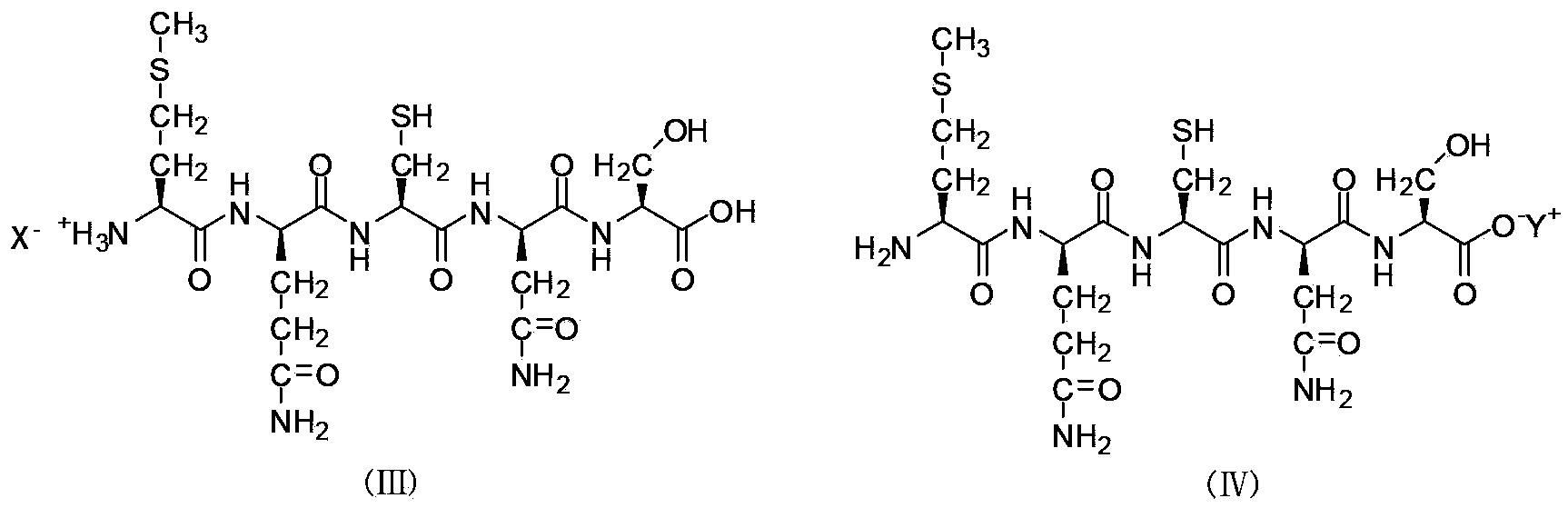
Liquid-phase synthesizing method for polypeptide
A liquid-phase synthesis and condensing agent technology, which is applied in the production of peptides and bulk chemicals, can solve problems such as undiscovered pentapeptide synthesis methods, and achieve the effects of easy control of the reaction process, simple production process, and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] a..Asn(Cbz)--Ser(tBu)--OtBu dipeptide preparation process:
[0059] Dissolve tBu-tert-butyl serine (63.5 g, 0.29 mol) and N-benzyloxycarbonyl (Cbz)-L-asparagine (78.0 g, 0.29 mol) in 1200 mL of organic solvent N,N-dimethylformamide In DMF, add condensing agent HOBT (43.6g, 0.32mol), DIC (40.6g, 0.32mol) and 122mL organic amine triethylamine, react at room temperature for 7 hours. After the reaction, the reaction solution was poured into 500 ml of saturated sodium bicarbonate solution and stirred for 10 minutes. After suction filtration, washing with water and drying, Asn(Cbz)-Ser(tBu)-OtBu (128 g) was obtained with a yield of 93.9%.
[0060] The NMR spectrum of Asn(Cbz)-Ser(tBu)-OtBu obtained is:
[0061] 1 H-NMR: (CDCl 3 ,300MHz,J:Hz)δδ7.41(d,1H,J=7.8),7.36-7.29(m,5H),6.49(d,1H,J=8.1),6.24(s,1H),5.76(s ,1H),5.60(m,1H),4.52-4.47(m,1H),3.75(dd,1H,J=3.0,8.7),3.50(dd,1H,J=2.7,8.7),2.88(dd, 1H, J=4.5, 15.6), 2.60 (dd, 1H, J=5.4, 15.6), 1.45 (s, 9H), 1.13 (s, 9H).
[...
Embodiment 2
[0083] a..Asn(Cbz)--Ser(tBu)--OtBu dipeptide preparation process:
[0084] tBu-serine tert-butyl ester hydrochloride (73.5g, 0.29mol) and N-benzyloxycarbonyl (Cbz)-L-asparagine (78.0g, 0.29mol) were dissolved in 1200mL DMF, and the condensing agent HOBT (43.6 g, 0.32mol), DIC (40.6g, 0.32mol) and 122mL triethylamine were reacted at room temperature for 7 hours. After the reaction, the reaction solution was poured into 500 ml of saturated sodium bicarbonate solution and stirred for 10 minutes. After suction filtration, washing with water and drying, Asn(Cbz)-Ser(tBu)-OtBu (130 g) was obtained.
[0085] b.. Remove the Cbz protecting group on the Asn (Cbz)--Ser (tBu)--OtBu dipeptide:
[0086] The above product Asn(Cbz)-Ser(tBu)-OtBu (130 g) was dissolved in 1500 mL of methanol, and 19.5 g of 10% Pd-C (10% by mass of Pd) was added. At room temperature, the reaction was hydrogenated for 2 hours, and the reaction was stopped. After filtering off Pd-C, the solvent was distilled of...
Embodiment 3
[0102] Salt-forming process of Met--Gln--Cys--Asn--Ser pentapeptide:
[0103] Take the pentapeptide Met-Gln-Cys-Asn-Ser (1.0 g), dissolve it in methanol-water (volume ratio, 1:1) solution, add 68.8 mg of sodium hydroxide, stir at room temperature for 2 hours, evaporate methanol under reduced pressure, The sodium salt of the pentapeptide Met-Gln-Cys-Asn-Ser (1.05 g) was obtained by lyophilization.
[0104]The preparation method of the acetic acid and trifluoroacetic acid salt of the pentapeptide Met-Gln-Cys-Asn-Ser is the same as above, that is, the Met-Gln-Cys-Asn-Ser pentapeptide is dissolved in methanol-water (volume ratio, 1:1) Add acetic acid or trifluoroacetic acid to the solution, stir at room temperature for 2 hours, evaporate methanol under reduced pressure, and freeze-dry to obtain acetate or trifluoroacetate of the pentapeptide Met-Gln-Cys-Asn-Ser.
[0105] It is also possible to react the Met-Gln-Cys-Asn-Ser pentapeptide with an acid or a base under the action of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com