Preparation method of thymopentin freeze-dried powder injection
A technology for freeze-dried powder injection and thymus, which is applied in the field of medicine, can solve the problems of long preparation cycle of thymopentin freeze-dried powder injection, poor product quality of thymopentin freeze-dried powder injection, high energy consumption and production cost, and achieves clarification. Good quality, shortened production cycle and low content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A preparation method of thymopentin freeze-dried powder injection, the method comprises the following steps:
[0023] 1) Weigh 40.0g of mannitol, dissolve it in about 300ml of freshly prepared (not more than 12 hours after preparation) water for injection at 20~30℃, weigh 1.0g of thymopentin, and use about 200ml of freshly prepared 20~30℃ water for injection Dissolve in water for injection, mix the above-mentioned two solutions, add water for injection to 500ml, and prepare a mixed solution of mannitol and thymopentin;
[0024] 2) Adjust the pH value of the mixed solution of mannitol and thymopentin to 6 with lactic acid with a mass concentration of 25%;
[0025] 3) Filter the solution after adjusting the pH value through a sterilized plate filter with a pore size of 0.22 μm and air tightness;
[0026] 4) Divide 0.5ml of the obtained filtrate into 3ml vials to obtain a semi-finished product, put it into a freeze dryer, and freeze-dry it. The steps are as follows:
[0...
Embodiment 2
[0032] A preparation method of thymopentin freeze-dried powder injection, the method comprises the following steps:
[0033] 1) Weigh 80.0g of mannitol, dissolve it in about 500ml of freshly prepared (not more than 12 hours after preparation) water for injection at 20~30℃, weigh 10.0g of thymopentin, and use about 500ml of freshly prepared 20~30℃ water for injection Dissolve in water for injection, mix the above-mentioned two solutions, add water for injection to 1000ml, and prepare a mixed solution of mannitol and thymopentin;
[0034] 2) Adjust the pH value of the mixed solution of mannitol and thymopentin to 7 with lactic acid with a mass concentration of 25%;
[0035] 3) Filter the solution after adjusting the pH value through a sterilized plate filter with a pore size of 0.22 μm and air tightness;
[0036] 4) Divide 1ml of the obtained filtrate into 3ml vials to obtain a semi-finished product, put it into a freeze dryer, and freeze-dry it. The steps are as follows:
[0...
Embodiment 3
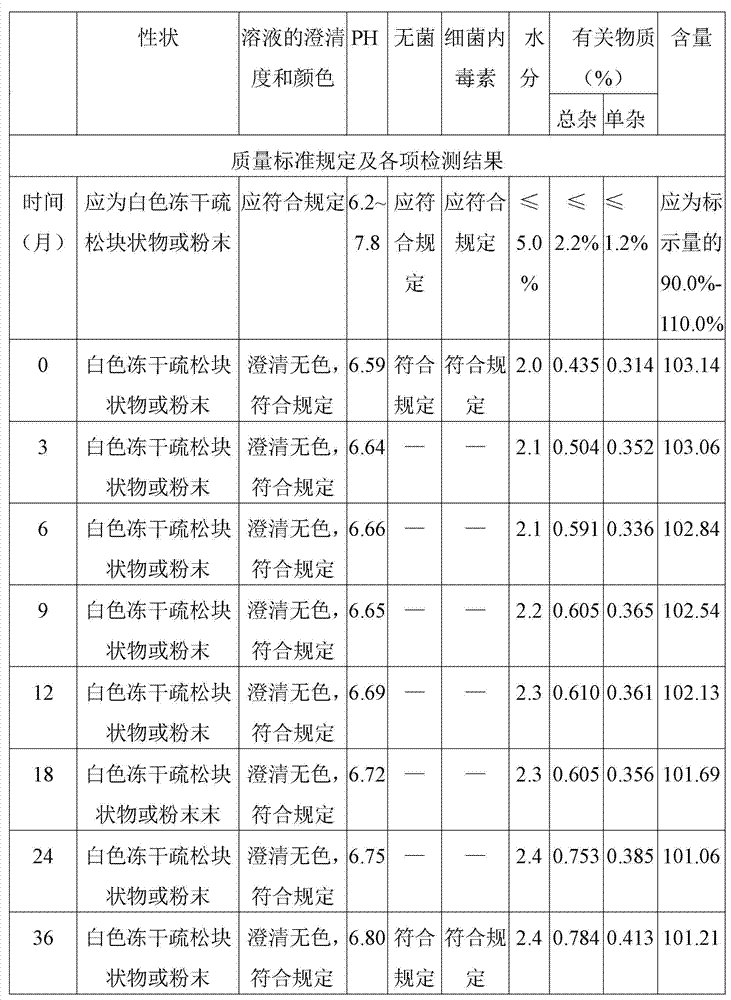
[0041] Embodiment 3 Effect comparison of different pH regulators and pH value control ranges
[0042] The present invention optimizes the optimal pH regulator and pH value by comparing the clarity of the product under the two factors of pH regulator and pH value control range in the batching stage, and comparing the product content and related substances under different pH regulators. conditions, the results are shown in Table 1 and Table 2:
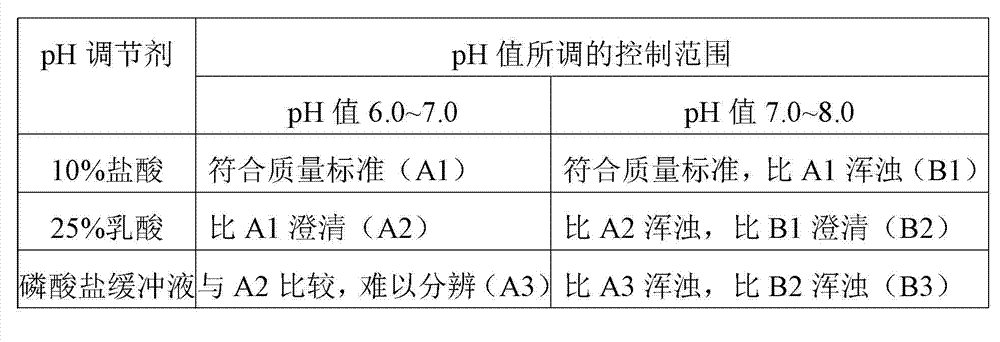
[0043] Table 1 Comparison of product clarity under different pH regulators and pH control ranges
[0044]
[0045] Note: The specific number of the solution is in parentheses.
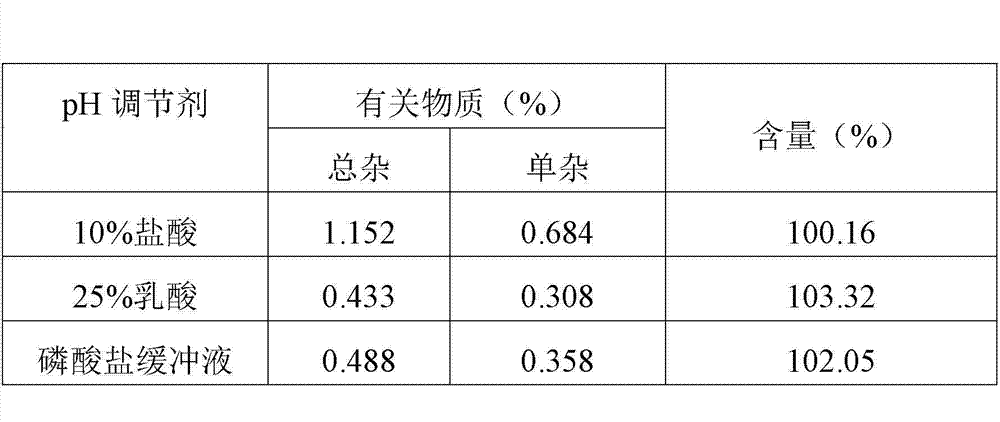
[0046] Table 2 Comparison of product content and related substances under different pH regulators
[0047]
[0048] Tests have shown that when 25% lactic acid is used in the batching stage to adjust the pH value to 6~7, the clarity of the thymopentin freeze-dried product produced is better, and when 25% lactic acid is used as the pH regulator, the related...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com