High-purity manganous nitrate and preparation method thereof
A technology of manganese nitrate and manganese sulfate solution, applied in the direction of manganese nitrate, etc., can solve the problem of high content of heavy metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
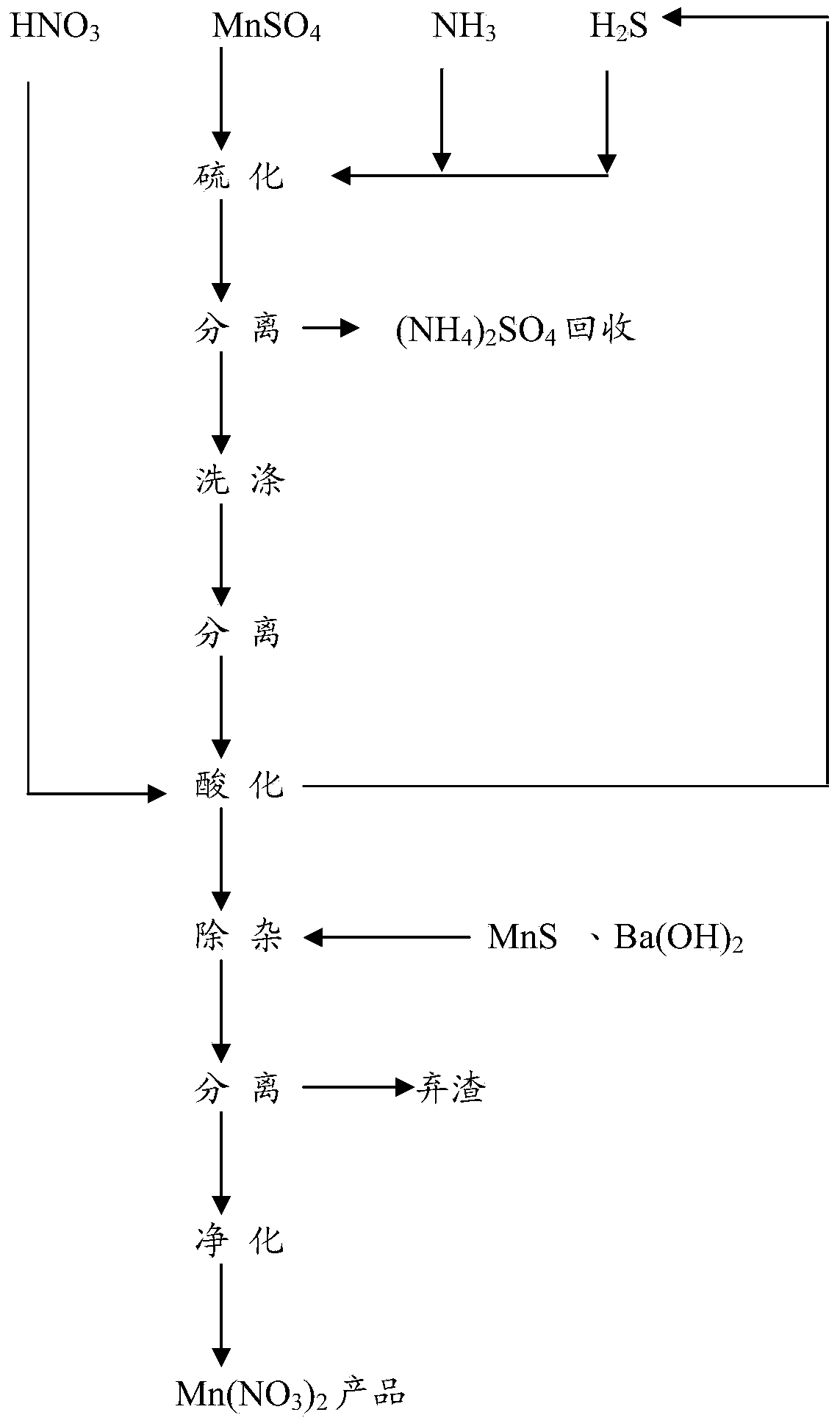
[0021] A preparation method of manganese nitrate, the method comprising:
[0022] (1) Vulcanization
[0023] Sulfurize the mixed gas containing ammonia and hydrogen sulfide gas with 150-300g / L (preferably 200-250g / L) manganese sulfate solution until the Mn in the solution 2+ When the concentration is less than 500ppm, stop the reaction, separate the solid from the liquid (preferably press filter), and then wash the obtained manganese sulfide filter cake for use. The filtrate obtained by solid-liquid separation is used to recover ammonium sulfate.
[0024] The manganese sulfate solution above can be made from commercially available manganese sulfate, or from flue gas desulfurization.
[0025] In a specific embodiment, ammonia gas and hydrogen sulfide gas (preferably in molar ratio of reaction) are passed into the absorption tower, and the manganese sulfate solution is sprayed to absorb the above-mentioned gases to carry out sulfidation reaction. The injection speed of the so...
Embodiment 1
[0048] (1) Vulcanization
[0049] The reaction molar ratio of ammonia gas and hydrogen sulfide gas is passed into the absorption tower, and then a manganese sulfate solution with a concentration of 150g / L is sprayed to absorb the above-mentioned gas for sulfidation reaction. When Mn 2+ When the concentration is less than 500ppm, the reaction is stopped, separated by pressure filtration, and the filtrate is recovered after treatment (NH 4 ) 2 SO 4 , The filter cake (manganese sulfide) is washed twice with 60°C deionized water according to the weight ratio of feed to water 1:4, each time for 3 hours, separated by pressing and filtering, and the water content of the filter cake is controlled to be less than 25% for later use.
[0050] (2) Acidification
[0051] Put the above filter cake in a glass-lined reaction pot, replace the air in the pot with nitrogen, then add 98% industrial grade nitric acid under airtight conditions, keep the temperature of the reaction system under ...
Embodiment 2
[0056] The reaction molar ratio of ammonia gas and hydrogen sulfide gas is passed into the absorption tower, and then a manganese sulfate solution with a concentration of 214g / L is sprayed to absorb the above-mentioned gas for sulfidation reaction. When Mn 2+ When the concentration is less than 500ppm, the reaction is stopped, separated by pressure filtration, and the filtrate is recovered after treatment (NH 4 ) 2 SO 4 , The filter cake (manganese sulfide) is washed twice with 62°C deionized water according to the weight ratio of feed to water 1:4, each time for 3 hours, separated by pressing and filtration, and the moisture content of the filter cake is controlled to be less than 25% for later use.
[0057] (2) Acidification
[0058] Put the above filter cake in a glass-lined reaction pot, replace the air in the pot with nitrogen, then add 98% industrial grade nitric acid under airtight conditions, keep the temperature of the reaction system under jacket cooling, stir the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com