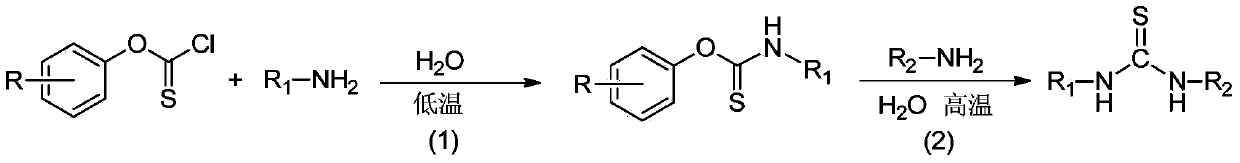
Method for realizing green synthesis of asymmetric thiocarbamide
A green synthesis and asymmetric technology, applied in organic chemistry methods, chemical instruments and methods, and the formation/introduction of functional groups, etc., can solve the problems of unsafe production, method limitations, rare raw materials, etc., and achieve convenient separation and purification, high yield Green environmental protection, the effect of easy availability of reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
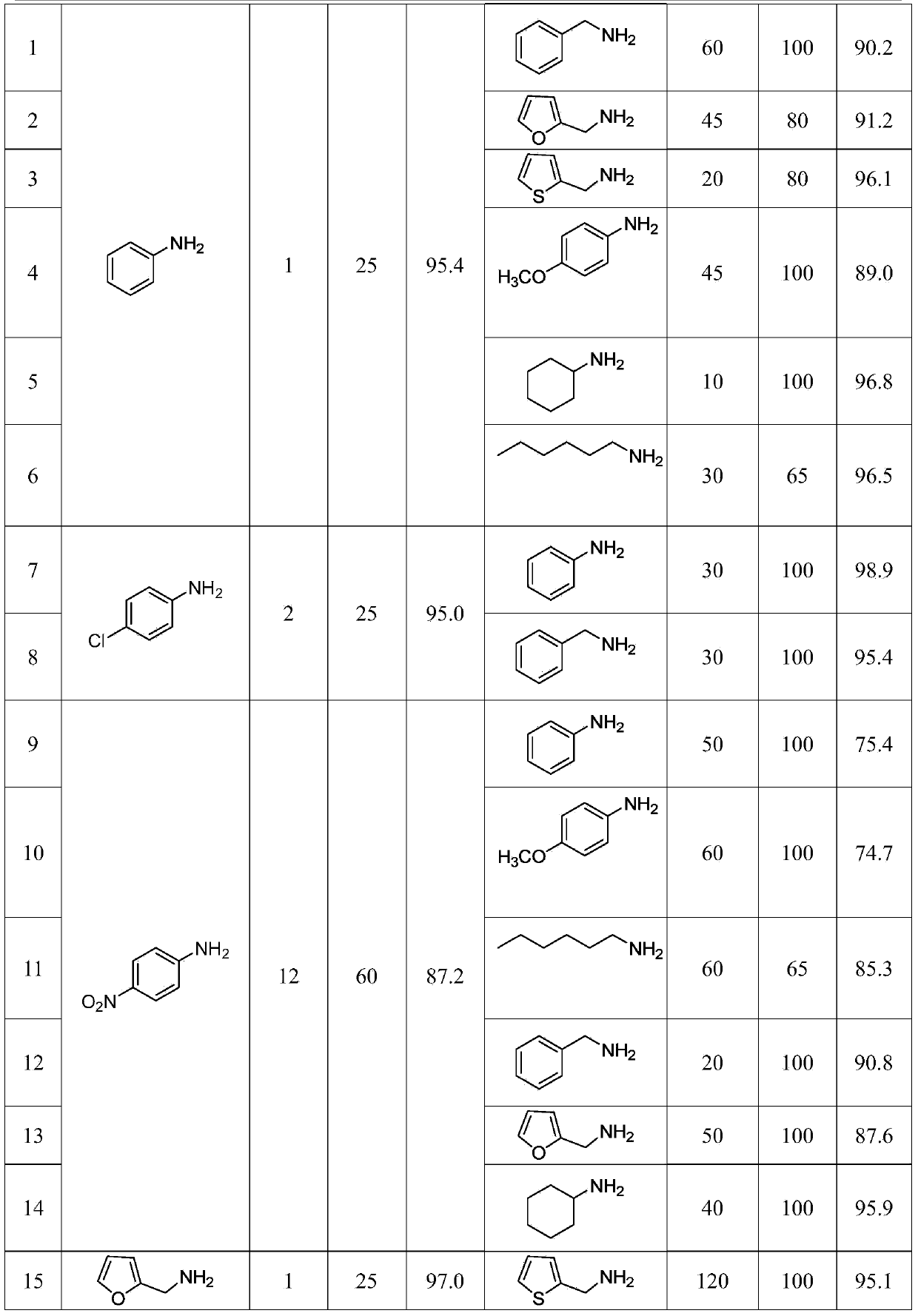
[0019] Step 1: Add phenoxythioacyl chloride (1mmol, 0.17g), 15mL of water and aniline (2mmol, 0.19g) sequentially into a 50mL one-necked flask, stir at 25°C for 1h, and stop the reaction. After suction filtration, the filter cake was washed successively with 10% hydrochloric acid and water to obtain a pure N-phenyl-O-phenylthioamide intermediate with a yield of 95.4%.
[0020] Step 2: Add N-phenyl-O-phenylthioamide (1mmol, 0.23g), 15mL of water and benzylamine (1mmol, 0.11g) sequentially into a 50mL single-necked flask, and stir at 100°C for 60min. Stop responding. After cooling and standing, suction filtration, the filter cake was washed successively with 10% hydrochloric acid and water to obtain phenylbenzylthiourea with a yield of 90.2%.
[0021] 1 HNMR(300MHZ,DMSO):δ=9.67(s,1H,NH),8.21(s,1H,NH),7.44(d,J=7.5Hz,2H,ArH),7.34-7.22(m,7H,ArH ),7.11(d,J=7.5Hz,1H,ArH),4.74(d,J=5.7Hz,2H,CH 2 ); 13 CNMR(75MHZ,DMSO):δ=181.2,139.7,139.5,129.1,128.7,127.9,127.3,124.7,123.8,47.6; M...
Embodiment 2
[0023] With reference to the method of Example 1, p-fluorophenoxysulfuryl chloride was used as the carbon sulfide reagent in the first step reaction, and the yield of the N-phenyl-O-phenylthioamide intermediate was 89.6%.
Embodiment 3
[0025] Referring to the method of Example 1, the molar ratio of phenoxythioacyl chloride and aniline in the first step reaction is 1:1, and the yield of N-phenyl-O-phenylthioamide intermediate is 85.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com