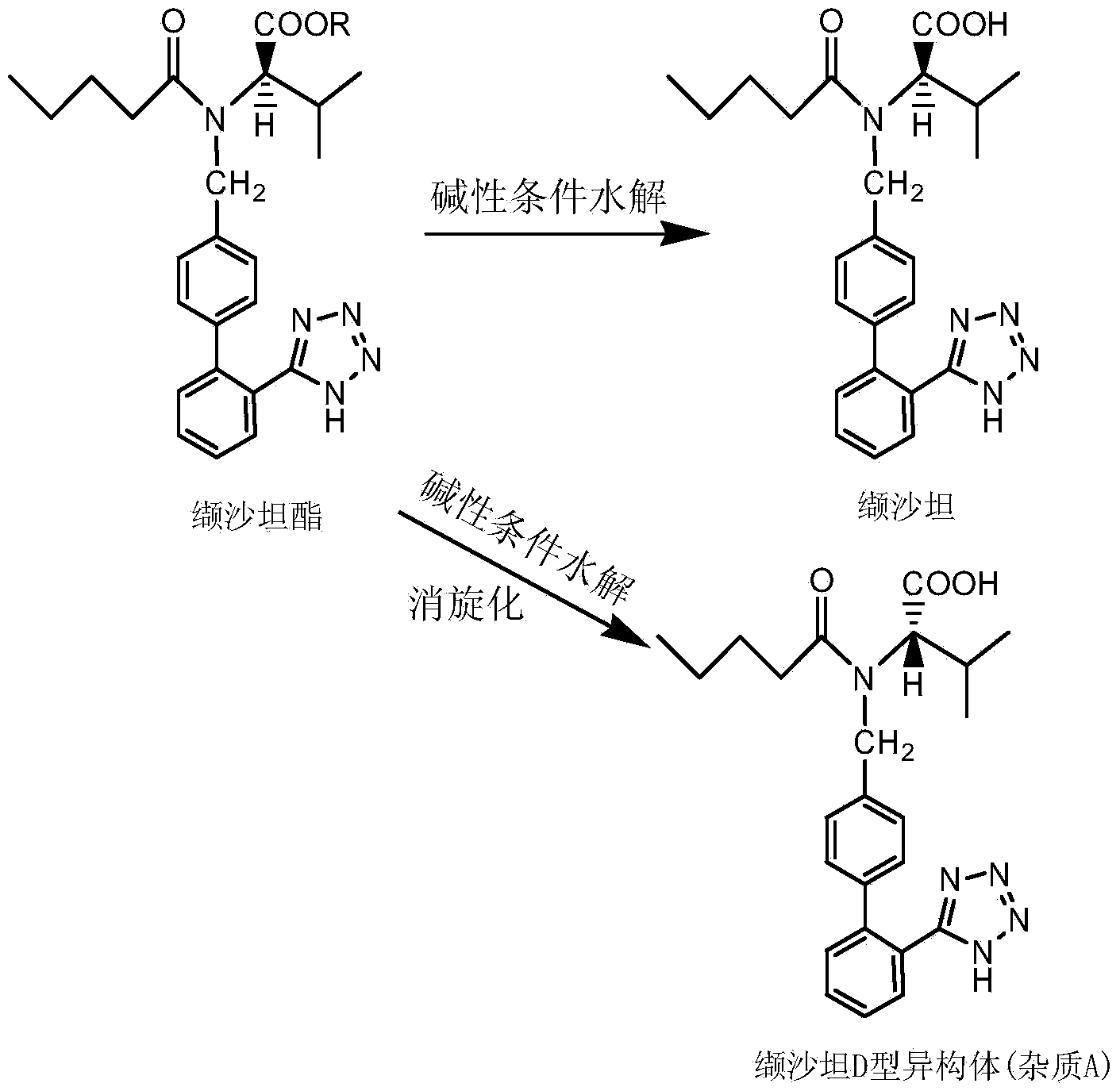
Valsartan refining method
A refining method and technology of valsartan, applied in the field of medicinal chemistry, can solve the problems of unsuitability for industrial production, unsatisfactory method and high price of butanone, and achieve the effects of easy control of process conditions, improved particle size and low solvent cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Dissolve 35g of valsartan (the content of D-isomer is 5.9%) in a mixed solvent of 35ml of ethanol and 120ml of ethyl acetate, and then heat to 45±2°C to completely dissolve the solid. Then slowly lower to room temperature, a large amount of white solid will be precipitated during the cooling process. Suction filtration after 4 hours gave 28.2 g of valsartan with a yield of 80.6% and a D-isomer content of 0.02%.
Embodiment 2
[0026] Dissolve 35g of valsartan (4.5% D-isomer content) in a mixed solvent of 16ml of methanol and 100ml of ethyl formate, and then heat to 40±2°C to completely dissolve the solid. Then slowly lower to room temperature, a large amount of white solid will be precipitated during the cooling process. Suction filtration after 4 hours gave 30.2 g of valsartan with a yield of 86.3% and a content of D-isomer of 0.01%.
Embodiment 3
[0028] Dissolve 35g of valsartan (the D-isomer content is 6.1%) in a mixed solvent of 25ml of isopropanol and 100ml of methyl acetate, and then heat to 65±2°C to completely dissolve the solid. Then slowly lower to room temperature, a large amount of white solid will be precipitated during the cooling process. Suction filtration after 4 hours gave 29.9 g of valsartan with a yield of 85.4% and a D-isomer content of 0.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com