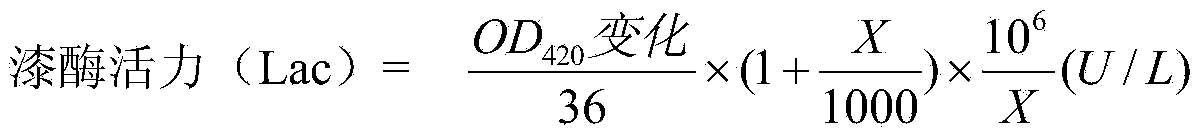Laccase modification method on basis of amino acid terminal carboxyl group and application thereof
A technology of terminal carboxyl group and amino acid, applied in the application field of modified laccase in the modification of regenerated plant fiber, to achieve the effect of improving laccase activity, reducing the amount of laccase used, and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
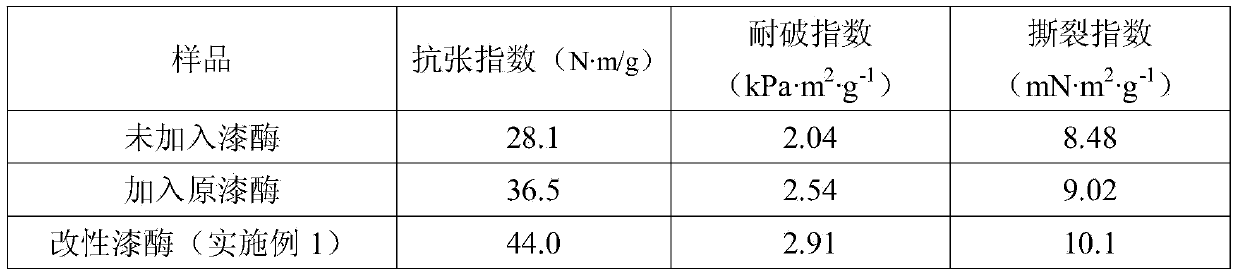
[0025] Modification step: Mix 5mL, 10mg / mL laccase solution (laccase SUKALacc produced by Aspergillus) with 0.4mg / mL, 5mL L-phenylalanine methyl ester hydrochloride solution, add 1.0 mg1-Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride 1-Ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride (EDC), reacted at 25°C for 4h under magnetic stirring conditions, Wherein the laccase solution and the L-phenylalanine methyl ester hydrochloride solution all use the potassium dihydrogen phosphate / dipotassium hydrogen phosphate (KH 2 PO 4 / K 2 HPO 4 ) buffer solution preparation. After the reaction is completed, transfer the mixed solution into a dialysis bag, and dialyze for 48 hours at room temperature in a dipotassium hydrogen phosphate / citric acid buffer solution with a pH of 5.0 and a concentration of 0.2 mol / L to remove excess L-phenylalanine methyl ester hydrochloride and EDC, to obtain a modified laccase solution, dilute to 25mL, freeze and store for later use, and ...
Embodiment 2
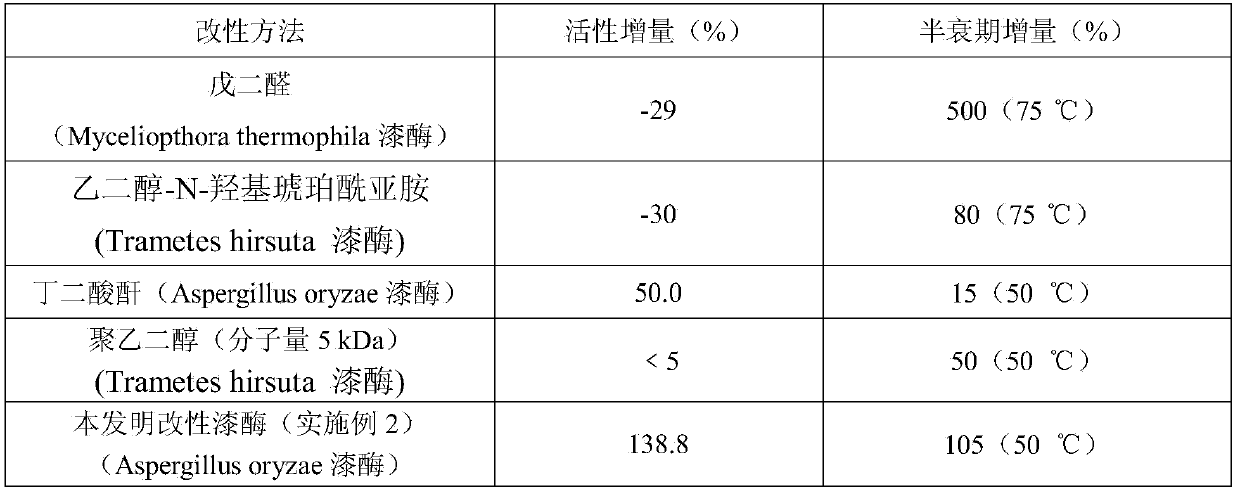
[0041]Mix 5 mL, 10 mg / mL laccase solution (laccase SUKALacc produced by Aspergillus) with 0.2 mg / mL, 6 mL L-phenylalanine methyl ester hydrochloride solution, and add 0.8 mg 1-ethyl-( 3-Dimethylaminopropyl) carbodiimide hydrochloride 1-Ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride (EDC), reacted at 25°C for 4h under magnetic stirring conditions, in which the laccase solution Both potassium dihydrogen phosphate / dipotassium hydrogen phosphate (KH 2 PO 4 / K 2 HPO 4 ) buffer solution preparation. After the reaction is completed, transfer the mixed solution into a dialysis bag, and dialyze at room temperature for 24 hours in a dipotassium hydrogen phosphate / citric acid buffer solution with a pH of 5.0 and a concentration of 0.2 mol / L to remove excess L-phenylalanine methyl Ester hydrochloride and EDC to obtain a modified laccase solution, dilute to 25mL, and freeze for future use. The laccase activity and half-life were determined according to the method described ...
Embodiment 3
[0044] Mix 5 mL, 10 mg / mL laccase solution (laccase SUKALacc produced by Aspergillus) with 0.1 mg / mL, 10 mL L-phenylalanine methyl ester hydrochloride solution, and add 1.0 mg 1-ethyl-( 3-Dimethylaminopropyl) carbodiimide hydrochloride 1-Ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride (EDC), reacted at 25°C for 4h under magnetic stirring conditions, in which laccase solution and L - Phenylalanine methyl ester hydrochloric acid solution is all used potassium dihydrogen phosphate / dipotassium hydrogen phosphate (KH 2 PO 4 / K 2 HPO 4 ) buffer solution preparation. After the reaction is completed, transfer the mixed solution into a dialysis bag, and dialyze at room temperature for 12 hours in a dipotassium hydrogen phosphate / citric acid buffer solution with a pH of 5.0 and a concentration of 0.2 mol / L to remove excess L-tryptophan methyl ester hydrochloride and EDC to obtain a modified laccase solution, which was adjusted to 25 mL, and stored frozen for later use. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com