Preparation method of aromatic ring-containing bridged silsesquioxane monomer
A technology of silsesquioxane and semisiloxane, which is applied in the field of preparation of aromatic ring bridged silsesquioxane monomers, and can solve problems such as difficult separation, difficult synthesis of precursors, and difficult availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
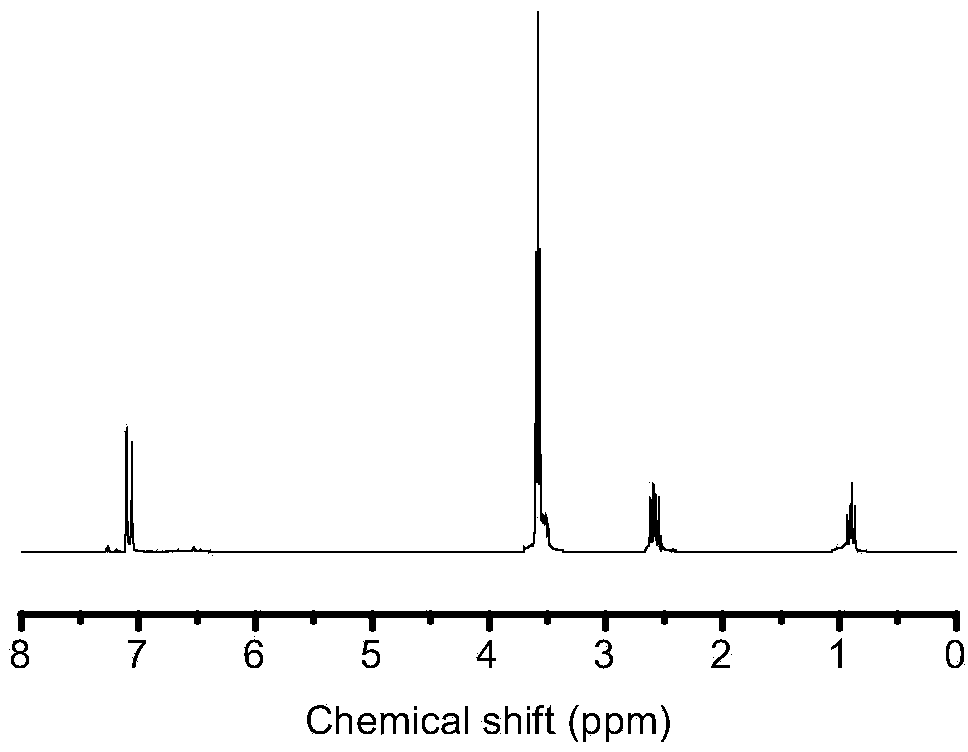
[0027] Embodiment 1: Preparation of 1,4-bis(trimethoxysilylethyl)benzene
[0028] In this embodiment, 1,4-bis(trimethoxysilylethyl)benzene was prepared according to the following steps:
[0029] a. Add 15 mg of catalyst Pt (dcp) to the three-necked flask, vacuumize for 15 minutes using the double-pipe system, and fill with nitrogen for 3 minutes. Repeat this three times so that there is no nitrogen atmosphere in the three-necked flask; use a syringe to draw 28 mL of sodium dewatered in advance1,4 - Divinylbenzene and 150 mL of tetrahydrofuran were injected into a sealed three-necked flask.
[0030] b. Add 150 mL of tetrahydrofuran into the three-necked flask that completed step a, and then add 60 mL of trimethoxysilane dropwise while stirring to obtain mixed liquid A.
[0031] c. The mixed liquid A obtained in step b was refluxed for 6 hours under the condition of stirring and heating in an oil bath at 70° C., stopped heating after the reaction was completed, and cooled to ro...
Embodiment 2
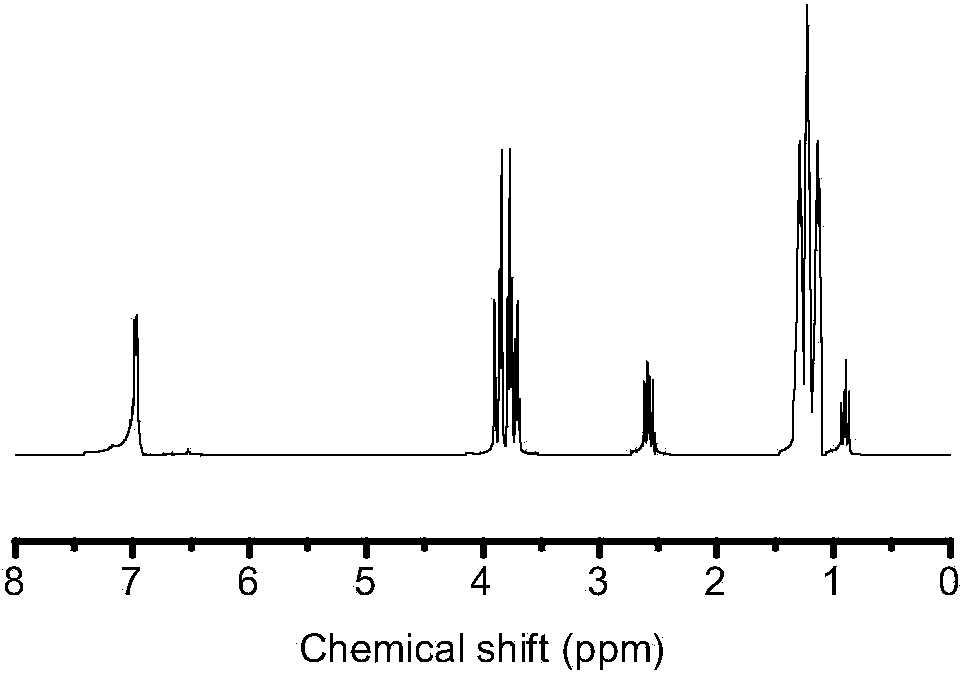
[0034] Embodiment 2: Preparation of 1,4-bis(triethoxysilylethyl)benzene
[0035] In this embodiment, 1,4-bis(triethoxysilylethyl)benzene is prepared according to the following steps:
[0036] a. Add 20 mg of catalyst Pt (dcp) to the three-necked flask, vacuumize for 15 minutes using the double-pipe system, and fill with nitrogen for 3 minutes. Repeat this three times, so that there is no nitrogen atmosphere in the three-necked flask; draw 28 mL of pre-dehydrated sodium with a syringe1,4 - Divinylbenzene, injected into a sealed three-necked flask.
[0037] b. Add 300 mL of tetrahydrofuran into the three-necked flask that completed step a, and then add 110 mL of triethoxysilane dropwise while stirring to obtain mixed liquid A.
[0038] c. The mixed liquid A obtained in step b was refluxed for 6 hours under the condition of magnetic stirring and heating in an oil bath at 70° C., stopped heating after the reaction was completed, and cooled to room temperature to obtain mixed liqu...
Embodiment 3
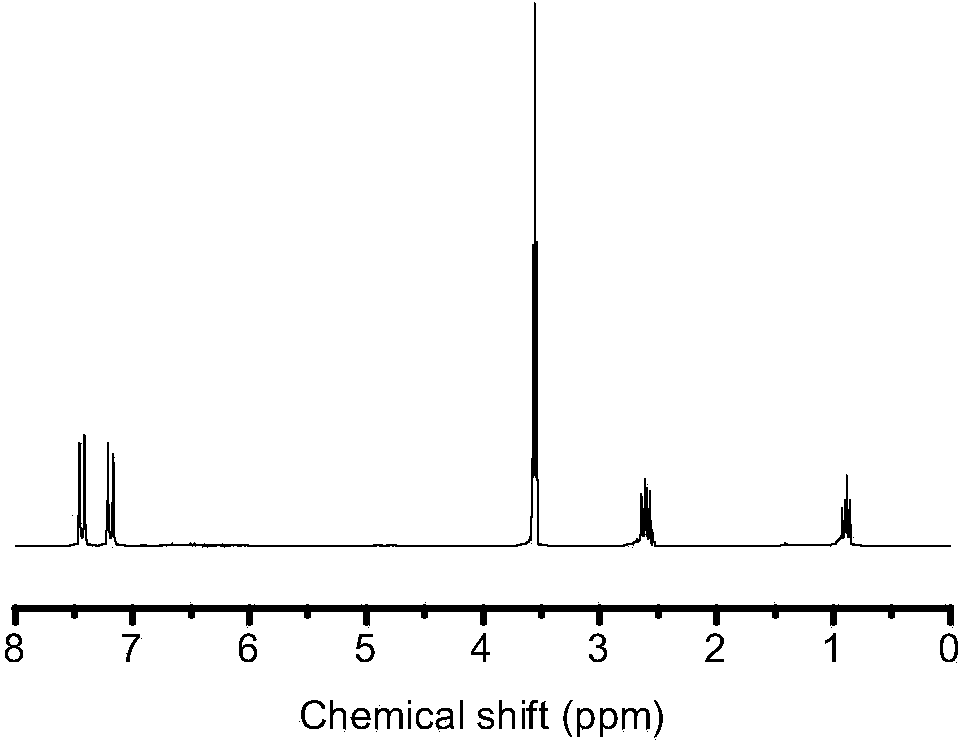
[0041] Example 3: Preparation of 4,4'-bis(trimethoxysilylethyl)biphenyl
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com