Carboxymethyl cellulose grafted polylactic acid amphiphilic polymer, as well as preparation method and application thereof
A carboxymethyl cellulose and amphiphilic polymer technology, which is applied in the fields of polymer chemistry and renewable resources, can solve the problems affecting the preparation of amphiphilic cellulose derivative nanomicelles, low degree of hydrophobization and controllability It can achieve the effect of good anti-dilution stability, process safety and high transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
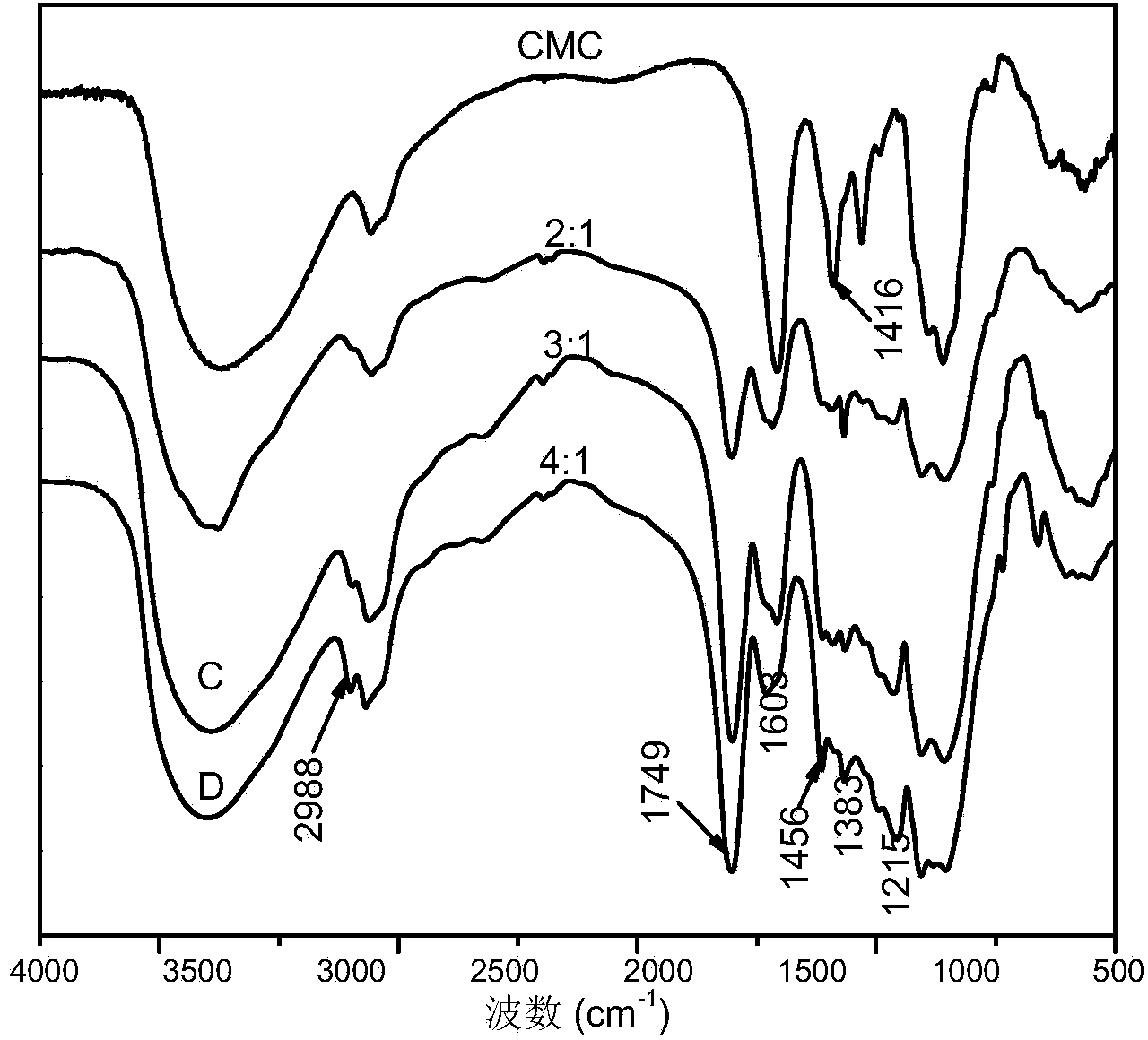
[0035] Accurately weigh 10g of chloride-1-allyl-3-methylimidazolium ionic liquid (AmimCl) into a 50ml three-neck flask, heat and dissolve in an oil bath at 80°C, add 1g of carboxymethylcellulose under stirring conditions, Introduce nitrogen, stir at 80°C for 8h, and heat up to 120°C after the carboxymethyl cellulose is dissolved. After the temperature is stable, add the reaction monomer lactide L-LA (the molar ratio of lactide to carboxymethyl cellulose basic sugar unit is 2:1) and stannous octoate (the mass percentage of lactide is 0.2% ), under nitrogen protection, and reacted for 24h under magnetic stirring (1000rpm). The reaction was terminated, and the temperature of the system was lowered to room temperature. Pour the mixture in the reactor into 250ml ethanol to make it precipitate, filter the precipitate, and wash 5 times with absolute ethanol to remove unreacted lactide, remaining catalyst and ionic liquid. The product was extracted in acetone at 75°C for 24h, and dr...
Embodiment 2
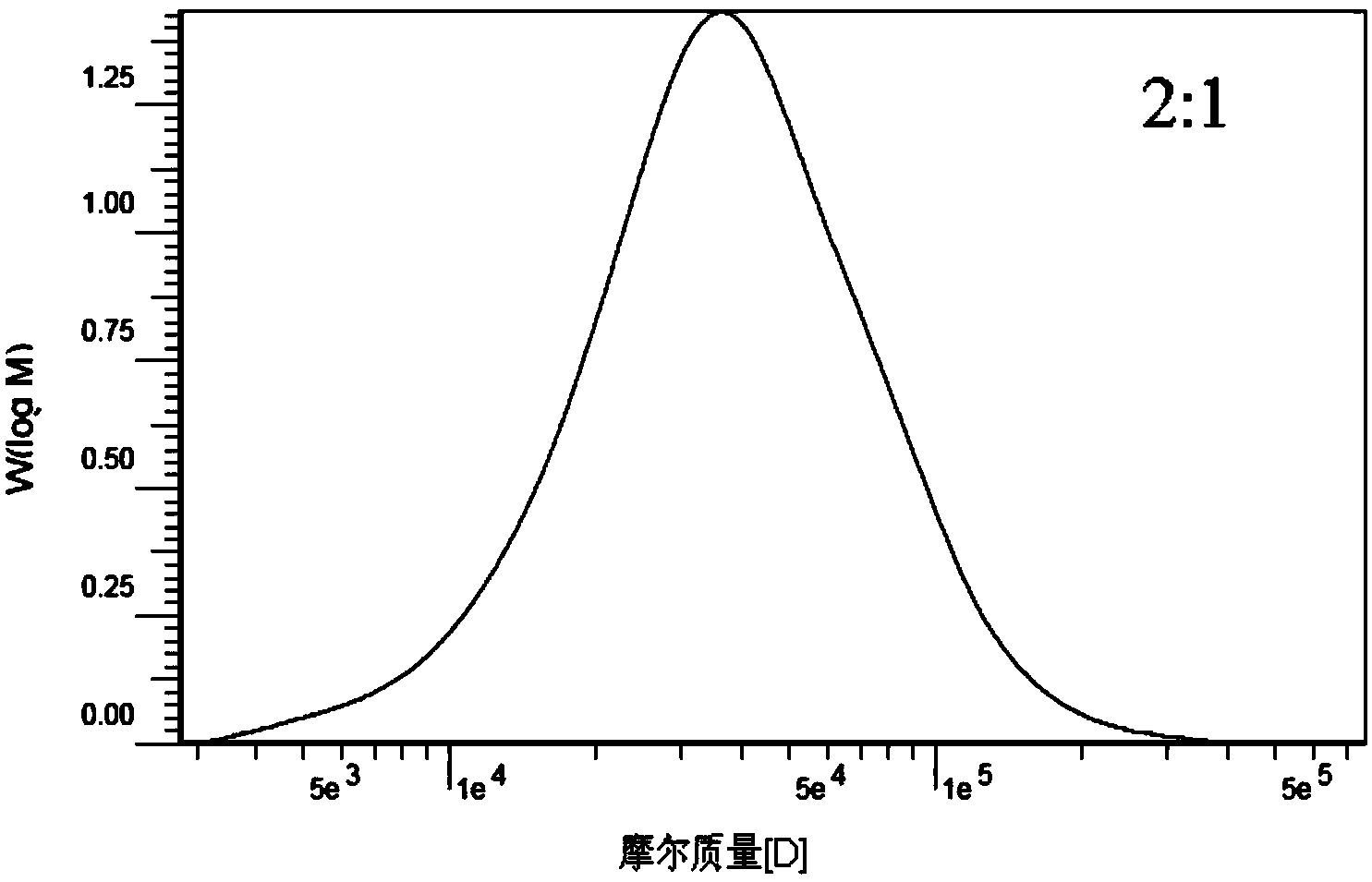
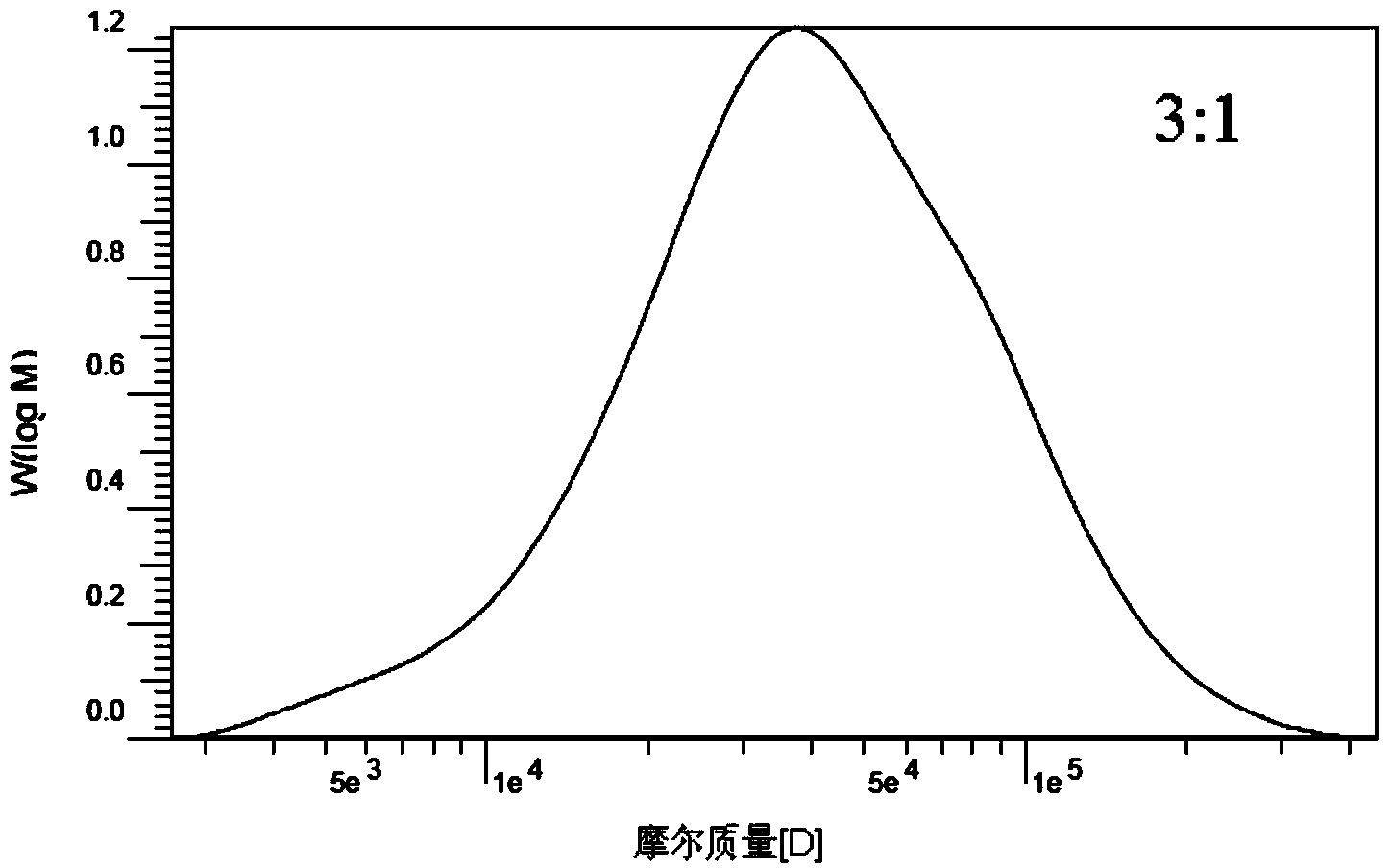
[0038] Accurately weigh 10g of chloride-1-allyl-3-methylimidazolium ionic liquid (AmimCl) (1-butyl-3-methylimidazolium chloride or 1-(2-hydroxyethyl)- 3‐Methylimidazolium chloride salt) was placed in a 50ml three-necked flask, heated and dissolved in an oil bath at 120°C, 2g of carboxymethylcellulose was added under stirring conditions, nitrogen was introduced, stirred at 120°C for 6h, and the carboxymethylcellulose After the element was dissolved, the temperature was raised to 120°C. After the temperature is stable, add the reaction monomer lactide L-LA (the molar ratio of lactide to carboxymethyl cellulose basic sugar unit is 3:1) and stannous octoate (the mass percentage of lactide is 0.3% ), under nitrogen protection, and reacted for 20h under magnetic stirring (1000rpm). The reaction was terminated, and the temperature of the system was lowered to room temperature. Pour the mixture in the reactor into 250ml ethanol to make it precipitate, filter the precipitate, and was...
Embodiment 3
[0041] Accurately weigh 10g of chloride-1-allyl-3-methylimidazolium ionic liquid (AmimCl) (1-butyl-3-methylimidazolium chloride or 1-(2-hydroxyethyl)- 3-Methylimidazolium chloride salt) was placed in a 50ml three-necked flask, heated and dissolved in an oil bath at 100°C, 1g of carboxymethylcellulose was added under stirring conditions, nitrogen was introduced, stirred at 100°C for 7h, and the carboxymethylcellulose After the element was dissolved, the temperature was raised to 130°C. After the temperature stabilizes, add the reaction monomer lactide L-LA (the molar ratio of lactide to carboxymethyl cellulose basic sugar unit is 4:1) and stannous octoate (the mass percentage of lactide is 0.4% ), under nitrogen protection, and reacted for 18h under magnetic stirring (1000rpm). The reaction was terminated, and the temperature of the system was lowered to room temperature. Pour the mixture in the reactor into 250ml ethanol to make it precipitate, filter the precipitate, and wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average particle size | aaaaa | aaaaa |
| Number average particle size | aaaaa | aaaaa |
| Number average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com