Preparation and application of human serum albumin-ruthenium inorganic medicine compound
A technology of human serum albumin and inorganic drugs, applied in the field of anti-tumor drugs, can solve problems such as toxic and side effects, achieve the effects of reducing toxicity, high vascular permeability and retention rate, and improving targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Preparation of human serum albumin-KP1019 ruthenium inorganic drug complex
[0024] KP1019 was prepared according to the literature: indazole (0.6g; 5.1mmol) was dissolved in 8ml of 12N HCl at 70°C, 2ml of Ru(Ⅲ) solution was added to the hot solution, stirred at 80-90°C for 15 minutes, and immediately appeared An ocher-colored substance appeared, continued stirring to remove HCl residues, filtered, washed with ethanol and ether, and dried in vacuo to obtain 0.296 g of the product, with a yield of 87%. The prepared KP1019 drug was weighed and prepared into a 100mM solution with DMSO, and human serum albumin (HSA) was prepared into a 1.5mM solution with secondary deionized water. The DMSO solution of KP1019 and the HSA aqueous solution were mixed at a ratio of 1:1, the content of DMSO was not more than 5%, and incubated at 4°C for 24 hours. Concentrate the mixture of HSA and ruthenium inorganic anti-cancer drugs, wash it repeatedly with deionized water twic...
Embodiment 2
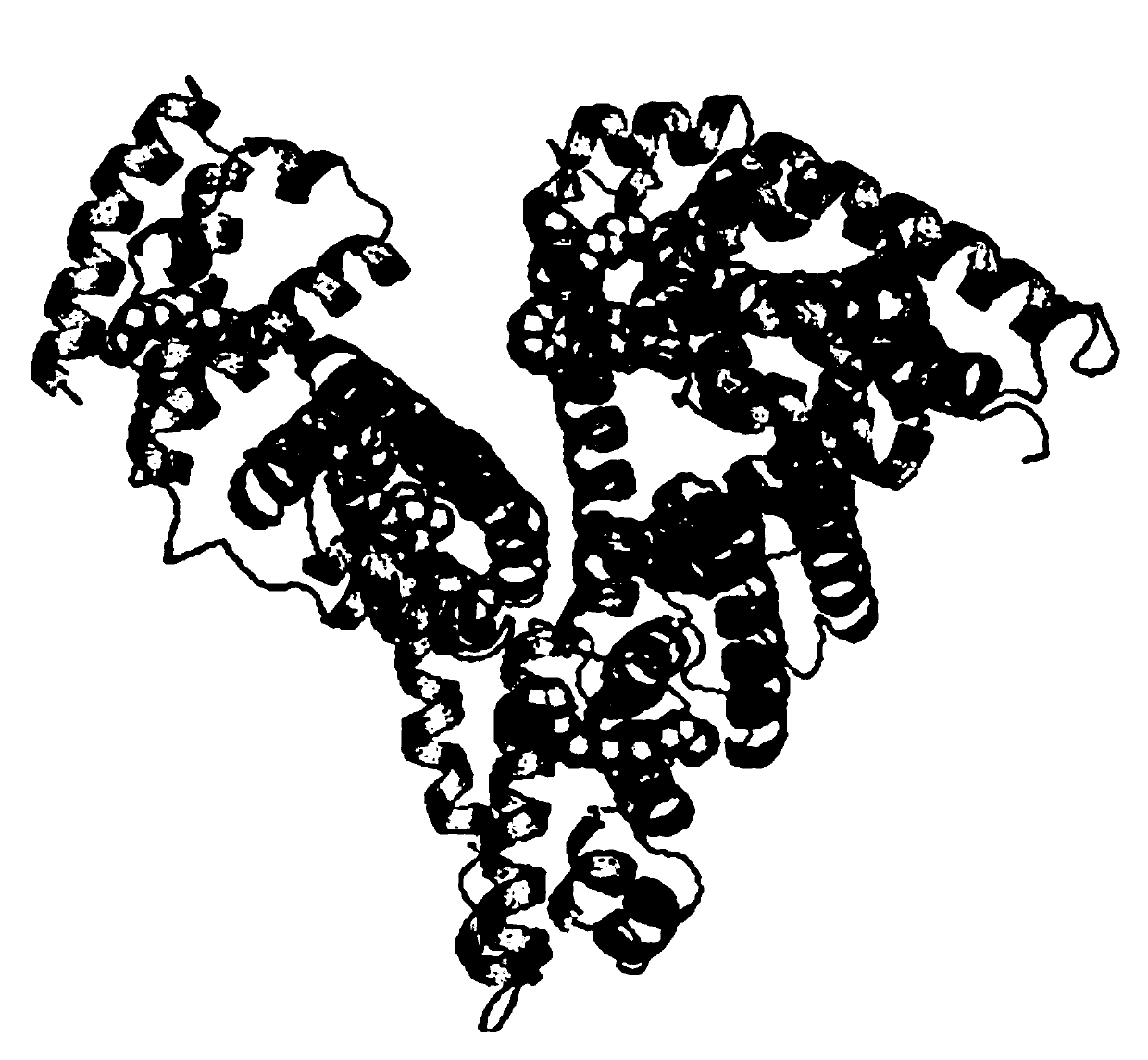
[0025] Embodiment 2: Structure determination of human serum albumin-KP1019 ruthenium inorganic drug complex
[0026] Mix HSA (1.5mM, 100μL), saturated hexadecanoic acid (2.5mM, 960μL), and drug (100mM, 1.5μL), incubate for 24 hours, centrifuge to remove the precipitate, then concentrate, wash repeatedly with deionized water twice, and finally Concentrate to 100 μL and incubate albumin suitable for X-ray diffraction by gas phase diffusion method (26-30% PEG3350, 5% glycerol, 5% 2-methyl-1,3-propanediol) in a sitting drop plate - Fatty acid-metal drug complex crystals. Crystal data were collected using the Shanghai Synchrotron Radiation Facility. After processing the data with HKL2000, use the molrep program of ccp4 to use the protein with strong homology with the amino acid sequence of the protein in the protein database as a template, quickly rotate and translate the function to find a suitable template, and integrate it. If there is no protein with strong homology in the ex...
Embodiment 3
[0028] Example 3: Effect of ruthenium inorganic drug / human serum albumin-KP1019 ruthenium inorganic drug complex on tumor cell proliferation in vitro
[0029] Digest the confluent monolayer cells with trypsin, collect the cells into the serum-containing medium, add 200 μl of cell suspension to each well of the tenth row in the middle of a flat-bottomed 96-well plate, and add 0.5×10 3 -10×10 3 cells, place the culture plate in CO 2 In the incubator, incubate in a humid environment at 37°C. After the cells adhere to the wall, add the above-mentioned different concentrations of ruthenium inorganic drugs prepared above and albumin carrier ruthenium inorganic drugs for 48 hours, and then add MTT (3-(4, 5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide), after continuing to cultivate for 4 hours, discard the supernatant, add 100ml of DMSO, and measure the optical density at 570nm on a microplate reader. Repeat 3 times in a row. The half inhibitory rate value of the ruthenium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com