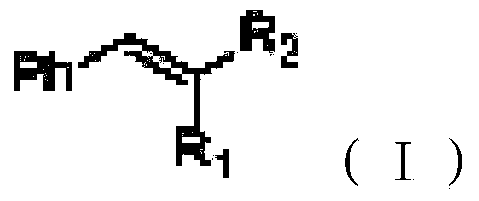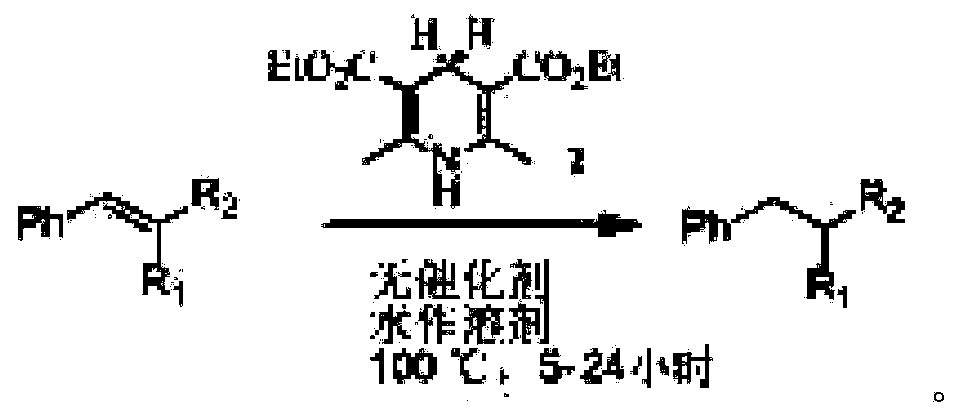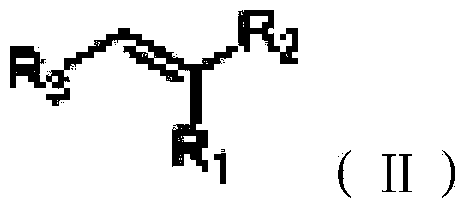Green conjugated double bond reduction method
A technology of conjugated double bonds and conjugated carbon-carbon double bonds, which is applied in the fields of organic reduction, chemical instruments and methods, and the preparation of organic compounds, to achieve the effect of high chemoselectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] The conjugated double bond compound 1 (2-styryl malononitrile, C 10 h 6 N 2 ) (30.8mg, 0.2mmol), dihydropyridinate (60.7mg, 0.24mmol) and 2mL of water were added to a single-mouthed 25mL chicken heart bottle, heated to 100°C, stirred for 24 hours, and extracted by adding 5mL of ethyl acetate (the same method operation three times), the organic layers were combined, dried over magnesium sulfate, evaporated under reduced pressure with a rotary evaporator, and then silica gel column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain 29.0 mg of the product, with a yield of 93%, without colored liquid.
[0025] H NMR spectrum: 1 HNMR (600MHz, CDCl 3 ): 3.28(d, J=6.96Hz, 2H), 3.91(t, J=6.96Hz, 2H), 7.32(d, J=6.72Hz, 2H), 7.38-7.42(m, 3H).
Embodiment 2
[0027]
[0028] The conjugated double bond compound 2 (2-(1-naphthylvinyl)-malononitrile, C 14 h 8 N 2 ) (40.8mg, 0.2mmol), dihydropyridinate (60.7mg, 0.24mmol) and 2mL of water were added to a single-mouthed 25mL chicken heart bottle, heated to 80°C, stirred for 24 hours, and extracted by adding 5mL of ethyl acetate (the same method operation three times), the organic layers were combined, dried over sodium sulfate, evaporated under reduced pressure with a rotary evaporator, and then silica gel column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain 30.1 mg of the product, with a yield of 73%, white solid.
[0029] Proton NMR spectrum: 1HNMR (400MHz, CDCl 3 ): 3.83(d, J=7.60Hz, 2H), 4.10(t, J=7.60Hz, 3H), 7.50-7.67(m, 4H), 7.88-7.98(m, 3H).
Embodiment 3
[0031]
[0032] The conjugated double bond compound 3 (2-(4-bromostyryl)-malononitrile, C 10 h 5 BrN 2 ) (46.4mg, 0.2mmol), dihydropyridinate (60.7mg, 0.24mmol) and 2mL of water were added to a single-mouthed 25mL chicken heart bottle, heated to 80 degrees, stirred for 24 hours, and extracted by adding 5mL of ethyl acetate (the same method operation three times), the organic layers were combined, dried over sodium sulfate, vacuum distillation with a rotary evaporator, and then silica gel column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain 35.1 mg of the product, a colorless liquid, and the yield 75%, 1HNMR (400MHz, CDCl 3 ): 3.27(d, J=6.80Hz, 2H), 3.93(d, J=6.76Hz, 1H), 7.23(d, J=8.32Hz, 2H), 7.57(d, J=8.40Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com