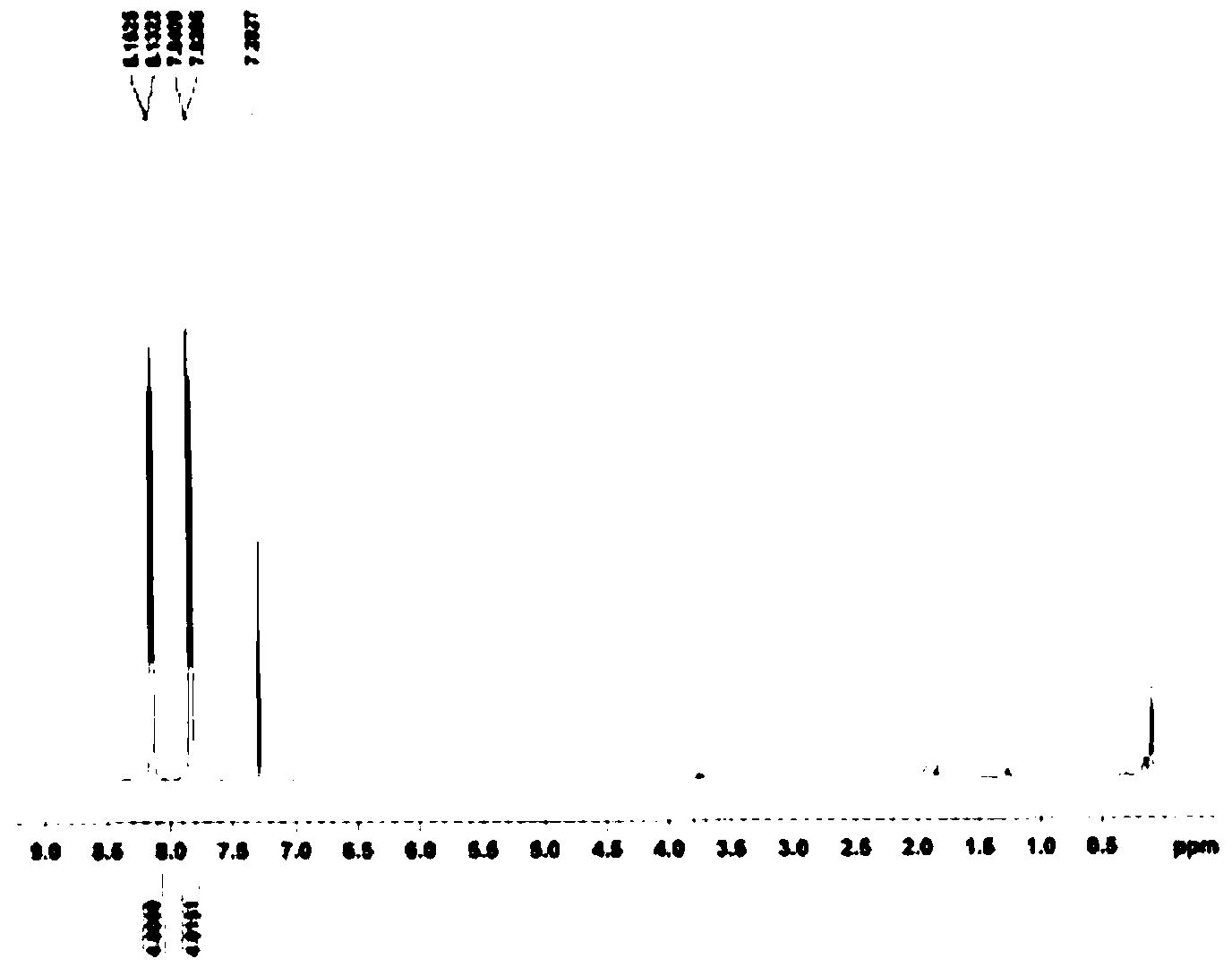
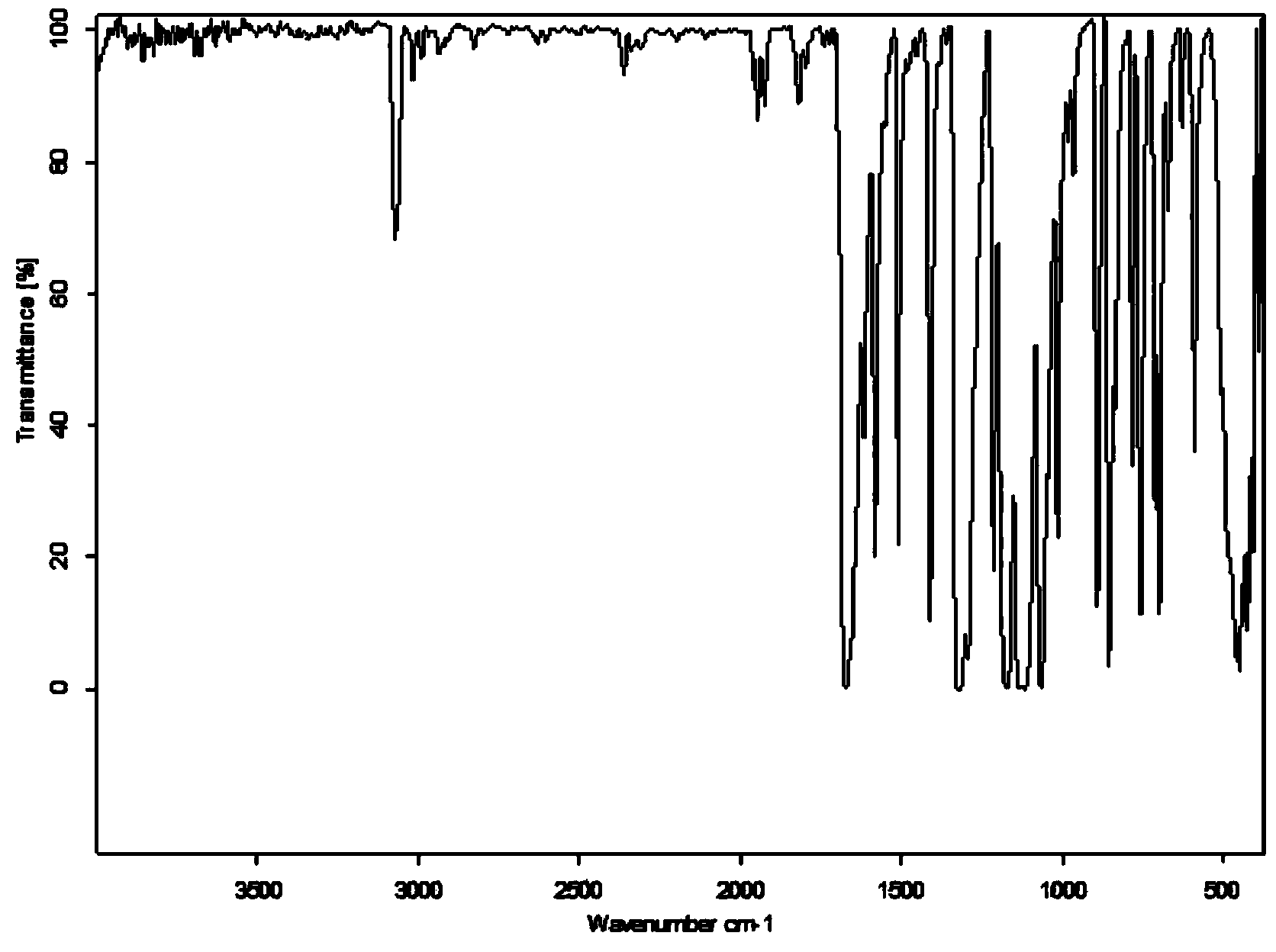
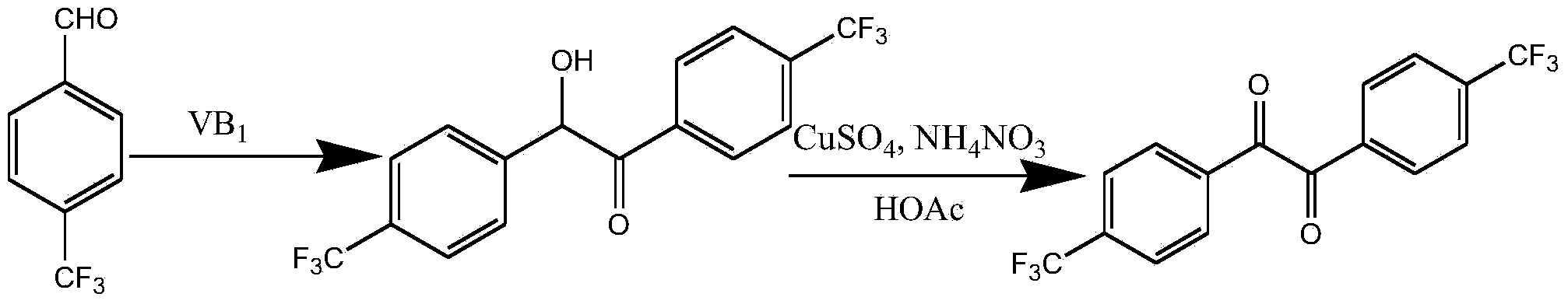
Preparation method of 4,4'-di-trifluoromethyl benzil
A technology of fluoromethylbenzyl and trifluoromethylphenyl, which is applied in the field of organic compound synthesis, can solve the problems of low yield, complicated catalyst preparation, long reaction time and the like, and achieves high product yield and easy reaction process. Control, the effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone
[0029] Under ice-water bath, add VB into the reactor successively 1 1.2g, 5.0mL distilled water, 15.0mL absolute ethanol and keep it for 15min, slowly add 3.3mol / L sodium hydroxide ethanol solution at 0~5℃ dropwise under stirring until the pH value of the solution is 8.0. Add 12.7g of p-trifluoromethylbenzaldehyde, adjust the pH value of the solution to 8.0 with 3.3mol / L sodium hydroxide ethanol solution at 0-5°C again, react at 60°C for 2h, cool in an ice-water bath, filter with suction, and filter the cake Wash with cold distilled water until the filtrate is neutral to give a white solid, which is recrystallized from ethanol to give 6.4 g of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone with a yield of 50.3% .
[0030] (2) Preparation of 4,4'-bistrifluoromethylbenzil
[0031] Add 6.4 g of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone, 3.0 g of ammonium nitrate, 1.0 g of anhydrous c...
Embodiment 2
[0037] (1) Preparation of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone
[0038] Under ice-water bath, add vitamin B to the reactor sequentially 1 2.9g, distilled water 10.0mL, absolute ethanol 32.0mL and keep it for 15min, slowly add 3.3mol / L sodium hydroxide ethanol solution at 0~5℃ dropwise under stirring until the pH value of the solution is 8.0. Add 25.5g of p-trifluoromethylbenzaldehyde, adjust the pH value of the solution to 8.0 with 3.3mol / L sodium hydroxide ethanol solution at 0-5°C again, react at 70°C for 3 hours, cool in an ice-water bath, filter with suction, and use for filter cake After washing with cold distilled water until the filtrate was neutral, a white solid was obtained, which was recrystallized from ethanol to obtain 13.7 g of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone with a yield of 53.7%.
[0039] (2) Preparation of 4,4'-bistrifluoromethylbenzil
[0040]Add 13.7g of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone, 8.0g of ammoniu...
Embodiment 3
[0042] (1) Preparation of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone
[0043] Under ice-water bath, add vitamin B to the reactor sequentially 1 1.2g, 5.0mL distilled water, 15.0mL absolute ethanol and keep it for 15min, slowly add 3.3mol / L sodium hydroxide ethanol solution at 0~5℃ dropwise under stirring until the pH value of the solution is 8.0. Add 12.7 g of p-trifluoromethylbenzaldehyde, adjust the pH value of the solution to 8.0 with 3.3 mol / L sodium hydroxide ethanol solution at 0-5°C again, react at 60-70°C for 4 hours, cool in an ice-water bath, and filter with suction. The filter cake was washed with cold distilled water until the filtrate was neutral to obtain a white solid, which was recrystallized from ethanol to obtain 6.6 g of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone with a yield of 51.7%.
[0044] (2) Preparation of 4,4'-bistrifluoromethylbenzil
[0045] Add 6.4 g of 1,2-bis(-4-trifluoromethylphenyl)-2-hydroxyethanone, 3.0 g of ammonium nit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com