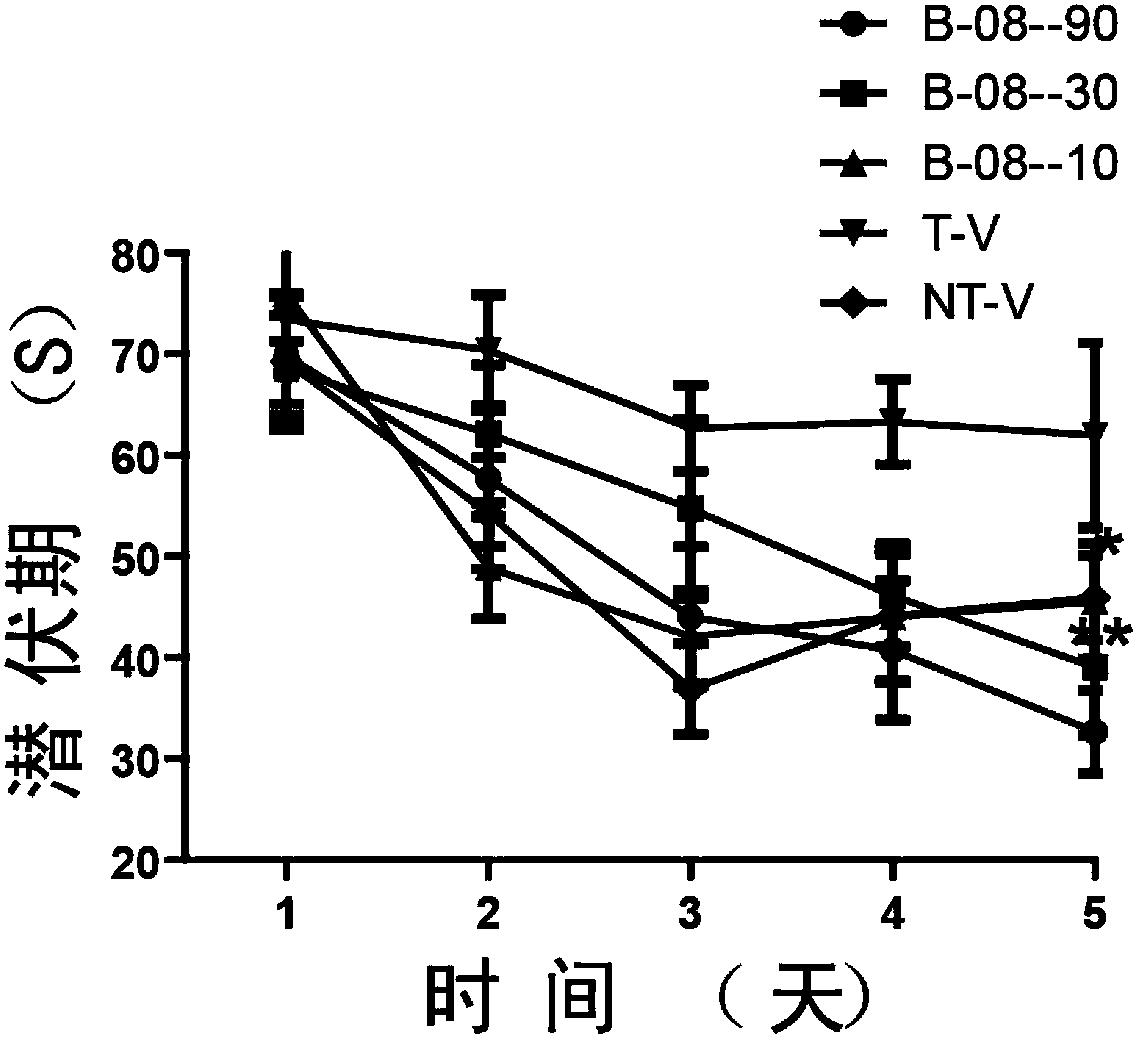
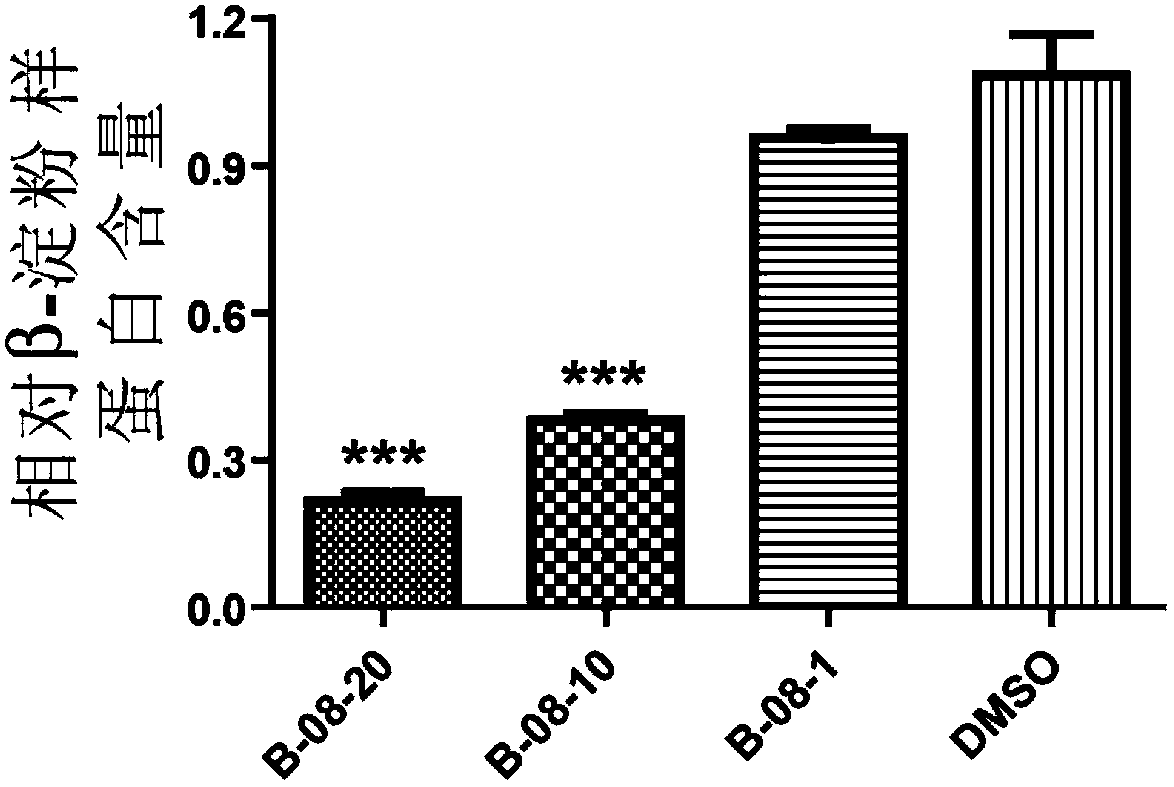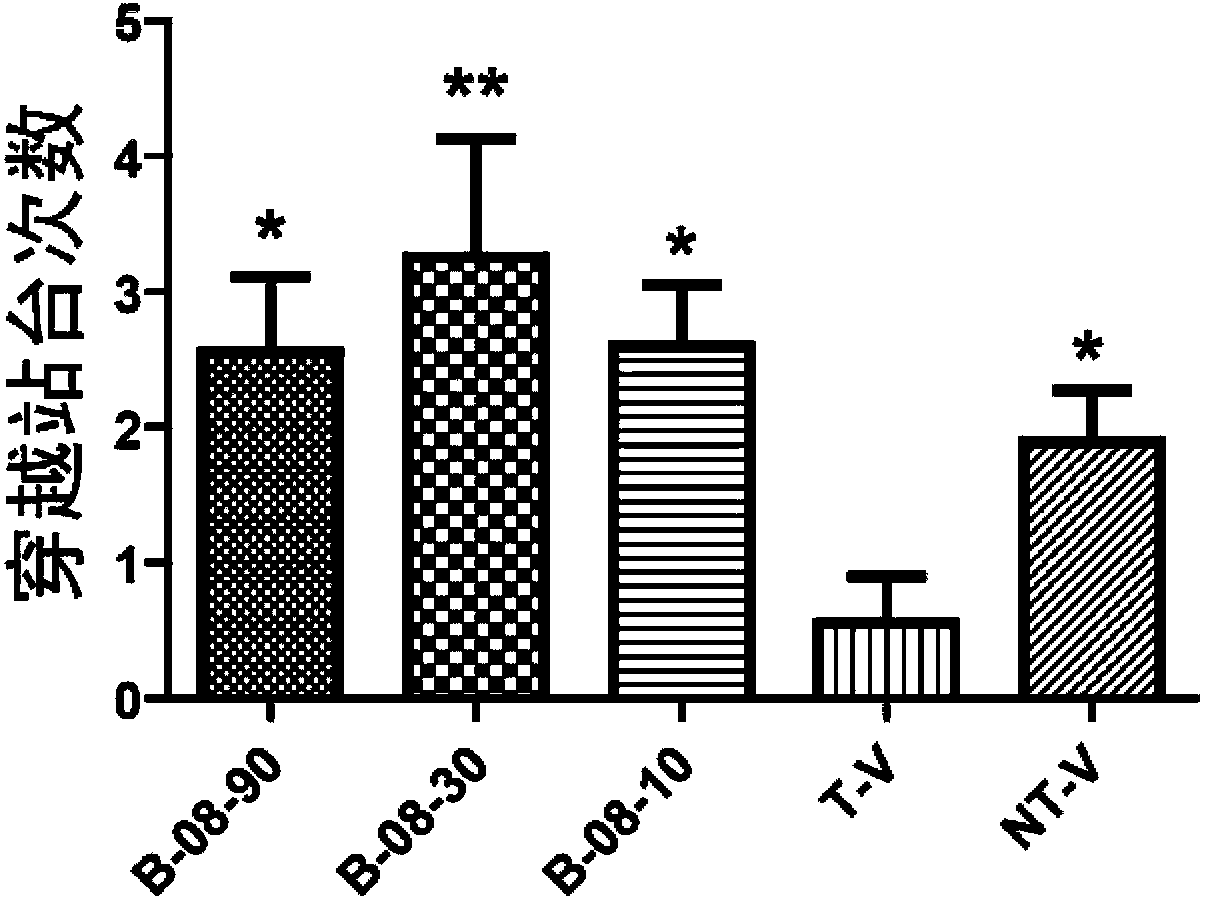Arctigenin carboxamide derivative, preparation method thereof, composition comprising arctigenin carboxamide derivative and uses thereof
The technology of aglycone carboxamide and arctium seed is applied in arctigen aglycone carboxamide derivative and preparation thereof, a composition comprising the derivative, and the field of use thereof, which can solve the problem of unseen arctigen aglycone, system structure Modified issues such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Preparation of compound A-01
[0062]
[0063] Under argon protection, dissolve arctigenin 1 (1mmol) in 10mL of anhydrous tetrahydrofuran, then add diisopropylethylenediamine (DIPEA, 1.5mmol), heat the mixture to 70°C, and add ethyl acetate. Isocyanate (2mmol), incubated for 24h. After the reaction, cool to room temperature, add 20 mL of dichloromethane, wash the organic phase with water (20 mL×2) twice, then with saturated brine (20 mL), dry with anhydrous magnesium sulfate, and filter. The solvent was removed from the filtrate under reduced pressure, and column chromatography (petroleum ether / acetone=3:1) was used to obtain compound A-01 as a white powder with a yield of 74%. 1 HNMR(300MHz, CDCl 3 )δ: 7.00 (d, J=8.0Hz, 1H), 6.80-6.71 (m, 2H), 6.65 (d, J=8.8Hz, 1H), 6.59-6.45 (m, 2H), 5.03 (s, 1H) ), 4.19-4.08 (m, 1H), 3.89-3.92 (m, 1H), 3.85 (s, 3H), 3.83 (s, 3H), 3.78 (s, 3H), 3.35 to 3.25 (m, 2H), 2.97(d,J=5.7Hz,2H),2.69-2.45(m,4H),1.21(t,J=7.2Hz,3H); ESI-...
Embodiment 2
[0064] Example 2: Preparation of Compound A-02
[0065]
[0066] Under argon protection, dissolve arctigenin (1mmol) in 10mL anhydrous tetrahydrofuran, then add diisopropylethylenediamine (DIPEA, 1.5mmol) and p-nitrophenyl chloroformate (1.5mmol) The mixture was heated to 70°C for 6 hours. Then, n-propylamine (2mmol) was added to the reaction solution, and the reaction was continued at 70°C for 10 hours. After the reaction, cool to room temperature, add 20 mL of dichloromethane, wash the organic phase with water (20 mL×2) twice, then with saturated brine (20 mL), dry with anhydrous magnesium sulfate, and filter. The solvent was removed from the filtrate under reduced pressure, and column chromatography (petroleum ether / acetone=3:1) was used to obtain compound A-02 as a white powder with a yield of 80%. 1 H NMR (300MHz, CDCl 3 )δ: 7.00(d,J=8.0Hz,1H),6.80-6.71(m,2H),6.65(d,J=8.0Hz,1H),6.58-6.48(m,2H),5.07(t,J =6.2Hz,1H), 4.16(dd,J=9.1,6.8Hz,1H), 3.90(d,J=8.0Hz,1H), 3.85(s,3H), 3....
Embodiment 3
[0067] Example 3: Preparation of Compound A-03
[0068]
[0069] Except that butyl isocyanate was used instead of ethyl isocyanate, compound A-03 was prepared in the same manner as in Example 1; silica gel column chromatography eluent: petroleum ether\acetone=3:1 column chromatography to obtain white powder The yield of compound A-03 was 72%. 1 H NMR (300MHz, CDCl3) δ: 6.99 (d, J=8.0Hz, 1H), 6.80-6.71 (m, 2H), 6.64 (d, J=8.0Hz, 1H), 6.57-6.47 (m, 2H) ,5.04(s,1H),4.14(dd,J=15.8,8.0Hz,1H),3.93-3.85(m,2H),3.86-3.73(m,9H),3.25(q,J=6.7Hz,2H ), 2.96(d,J=5.7Hz,2H),2.70-2.46(m,4H),1.45-1.33(m,2H),0.94(t,J=7.2Hz,3H); ESI-MS(m / z):472.3[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com