A method for preparing a pharmaceutical preparation in the form of an antioxidant-free solution containing pemetrexed or a salt thereof for injection
The technology of a pharmaceutical preparation and pemetrexed is applied in the field of pharmaceutical preparations in the form of an antioxidant-free solution containing pemetrexed or a salt thereof for injection, and can solve the problems of low stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of a solution for injection by distillation degassing
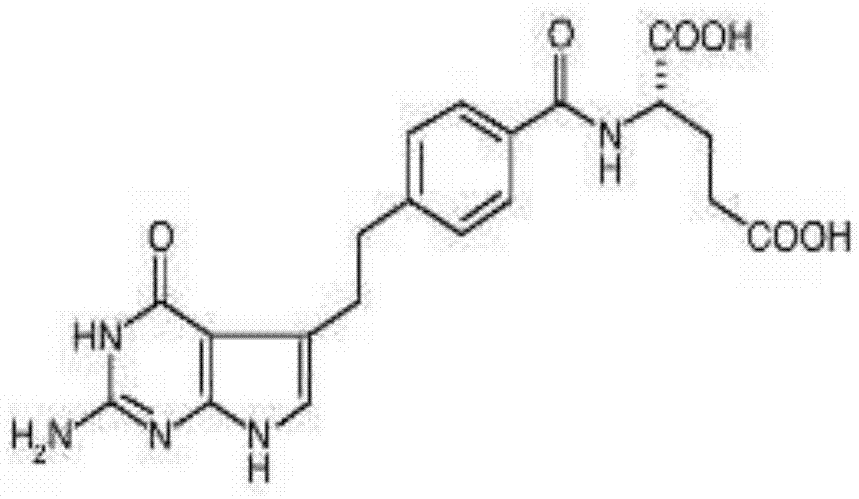
[0034]700 ml of water for injection was distilled by heating under a nitrogen atmosphere by heating, and then cooled under a nitrogen atmosphere to remove dissolved oxygen therein. The obtained dissolved oxygen concentration in the water for injection (deaerated water) was 0.5 ppm. Disodium pemetrexed (equivalent to 1.25 g pemetrexed) and 0.45 g sodium chloride were placed in a 100 ml glass container which was then placed in a glove bag. Also place the glass vial, rubber stopper, and sterile filter into the glove bag. Calibrate the electrode of the pH meter, wash it, and place it in the glove bag. Nitrogen gas was injected into the glove pocket under reduced pressure in order to adjust the partial pressure of oxygen to 0.1% v / v. Take 50ml of degassed water with a syringe and add it to a 100ml glass container in the glove bag to dissolve the components. The pH in the glove pocket was measu...
Embodiment 2
[0035] Example 2: Preparation of a solution for injection by vacuum degassing
[0036] Add pemetrexed disodium (equivalent to 2.5 g of pemetrexed) and 0.9 g of sodium chloride into a 250 ml glass bottle equipped with an air inlet and a vacuum exhaust port on its cap, and then add the 100ml of water to dissolve the components. The dissolved oxygen concentration in the water used for injection was 6.5 ppm. Connect nitrogen to the gas inlet, and connect a diaphragm vacuum pump to the vacuum exhaust. Repeated vacuum degassing operation and nitrogen purging. An aliquot of the resulting solution was used to measure the dissolved oxygen concentration and pH therein. As a result of the measurement, the dissolved oxygen concentration was 0.8 ppm, and the pH was 7.68.
[0037] Take the resulting solution by syringe and place it in the glove bag. Also place the glass vial, rubber stopper, and sterile filter into the glove bag. Nitrogen gas was injected into the glove belt under red...
experiment example
[0042] Experimental example: Stability test
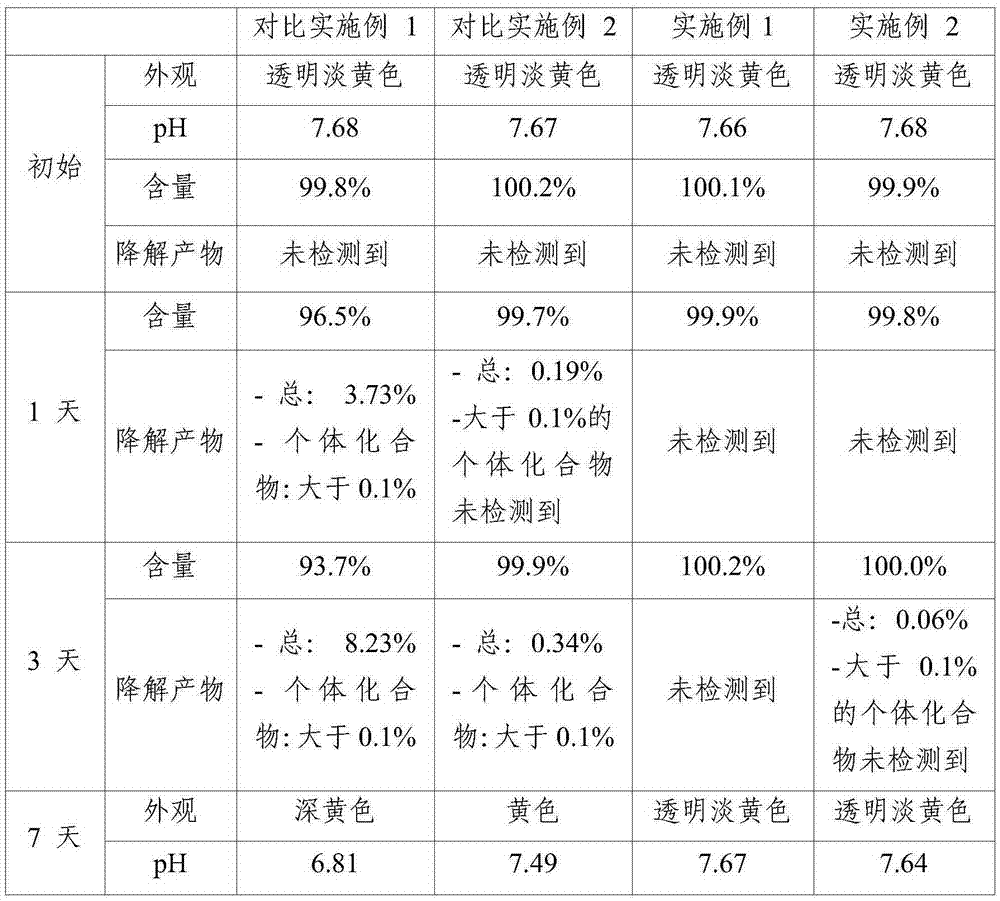
[0043] Under pressure conditions (75±2° C., relative humidity 85±5%), the stability of the solutions for injection prepared according to Examples and Comparative Examples was evaluated. The content of pemetrexed and degradation products was analyzed by high performance liquid chromatography (HPLC) under pressure conditions according to known methods published in Chromatography (Chromatographia, 2007, 66, pp. The results of the 7-day stability test are shown in Table 2 below.
[0044]
[0045]
[0046]
[0047] As can be seen from Table 2, in the solution for injection (prepared by using water for injection not adjusted for dissolved oxygen) according to the comparative example, even if it was not sterilized in an autoclave, when in When the solution for injection was stored under pressure for 1 week, a change in appearance and an increase in degradation products were clearly observed. In contrast, in the solutions for inj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com