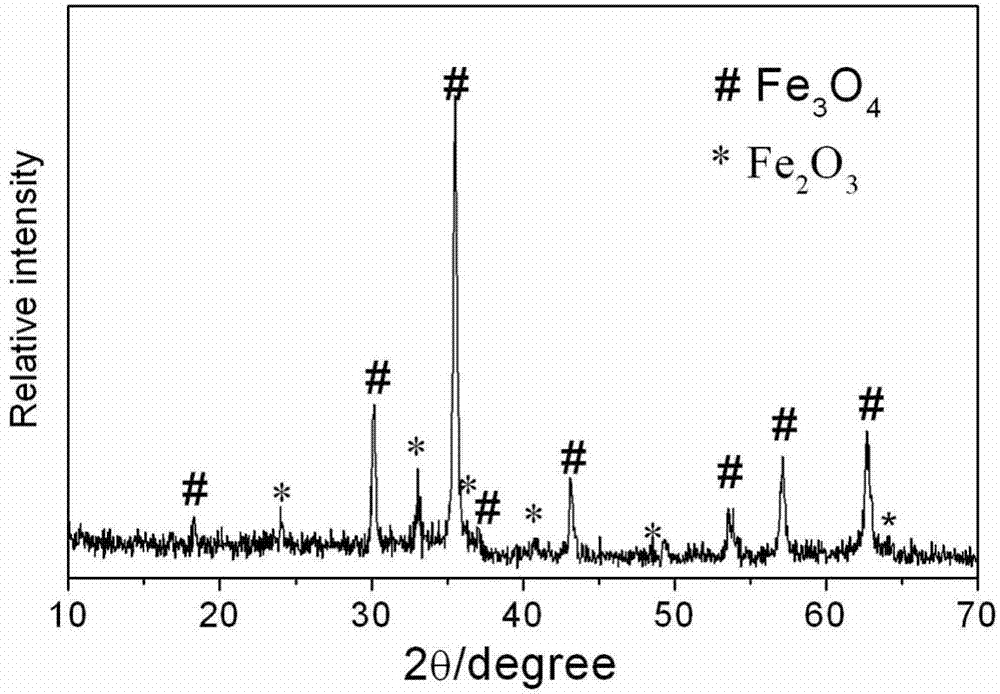
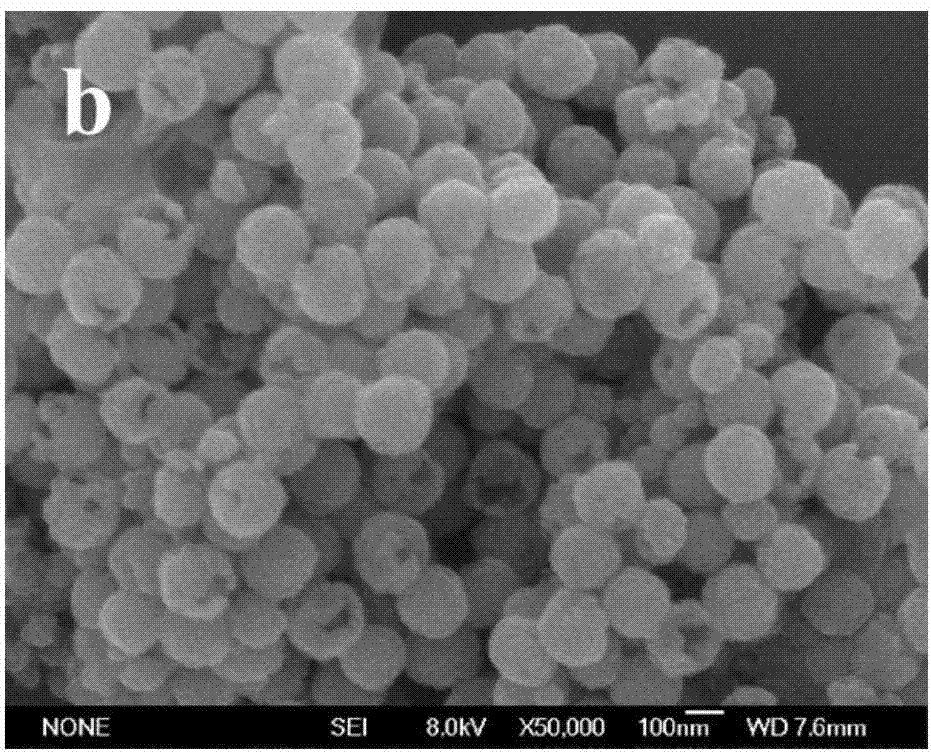
Preparation method of Fe3O4/alpha-Fe2O3 magnetic microspheres in core/shell structure
A technology of magnetic microspheres and core-shell structure, applied in the preparation of magnetic microspheres with core-shell structure and Fe3O4/α-Fe2O3 core-shell structure magnetic microspheres, can solve the problems of strict low-temperature burning process and achieve Simple method, wide application prospect, novel structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Weigh 1.5mmol FeSO 4 ·7H 2 O was dissolved in ethylene glycol, and after dissolution, 0.8g of PVP and 3.5mmol of urea were added, stirred to completely dissolve, and a light green solution was obtained.
[0020] (2) Transfer the above solution to a stainless steel reaction kettle lined with polytetrafluoroethylene, seal it, react at 200°C for 24h, and cool it down to room temperature naturally;
[0021] (3) The obtained precipitate was centrifuged and washed repeatedly with water and ethanol to obtain Fe 3 o 4 nanosphere dispersion;
[0022] (4) Weigh 0.037mmol FeCl 3 ·6H 2 O was dissolved in deionized water, and then 0.1 g of PVP and 0.33 mmol of urea were added in sequence, and stirred to completely dissolve to ensure that the reactants were evenly mixed, and then the prepared Fe 3 o 4 Microspheres, after reacting at 90°C for 24h, naturally cool to room temperature;
[0023] (5) Centrifuge the obtained precipitate, wash repeatedly with water and ethanol, a...
Embodiment 2
[0025] (1) Weigh 1.5mmol FeSO 4 ·7H 2 O was dissolved in ethylene glycol, and after dissolution, 0.8g of PVP and 3.5mmol of urea were added, stirred to completely dissolve, and a light green solution was obtained.
[0026] (2) Transfer the above solution to a stainless steel reaction kettle lined with polytetrafluoroethylene, seal it, react at 200°C for 24h, and cool it down to room temperature naturally;
[0027] (3) The obtained precipitate was centrifuged and washed repeatedly with water and ethanol to obtain Fe 3 o 4 nanosphere dispersion;
[0028] (4) Weigh 0.074mmol FeCl 3 ·6H 2 O was dissolved in deionized water, and then 0.1 g of PVP and 0.33 mmol of urea were added in sequence, and stirred to completely dissolve to ensure that the reactants were evenly mixed, and then the prepared Fe 3 o 4 Microspheres, after reacting at 90°C for 24h, naturally cool to room temperature;
[0029] (5) Centrifuge the obtained precipitate, wash repeatedly with water and ethanol, a...
Embodiment 3
[0031] (1) Weigh 1.5mmol FeSO 4 ·7H 2 O was dissolved in ethylene glycol, and after dissolution, 0.8g of PVP and 5.0mmol of urea were added, stirred to completely dissolve, and a light green solution was obtained.
[0032] (2) Transfer the above solution to a stainless steel reaction kettle lined with polytetrafluoroethylene, seal it, react at 200°C for 16 hours, and cool it down to room temperature naturally;
[0033] (3) The obtained precipitate was centrifuged and washed repeatedly with water and ethanol to obtain Fe 3 o 4 nanosphere dispersion;
[0034] (4) Weigh 0.05mmol FeCl 3 ·6H 2 O was dissolved in deionized water, and then 0.1 g of PVP and 0.33 mmol of urea were added in sequence, and stirred to completely dissolve to ensure that the reactants were evenly mixed, and then the prepared Fe 3 o 4 Microspheres, after reacting at 90°C for 12h, naturally cool to room temperature;
[0035] (5) Centrifuge the obtained precipitate, wash repeatedly with water and ethano...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com