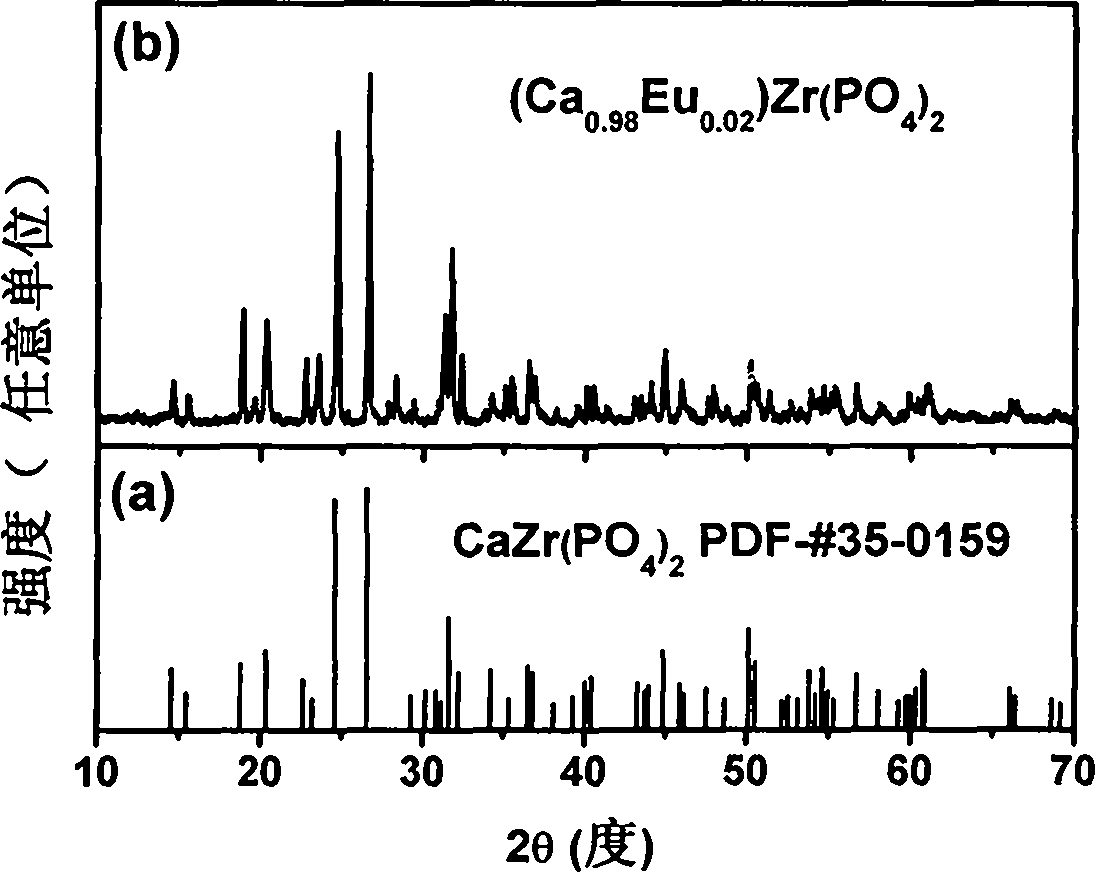
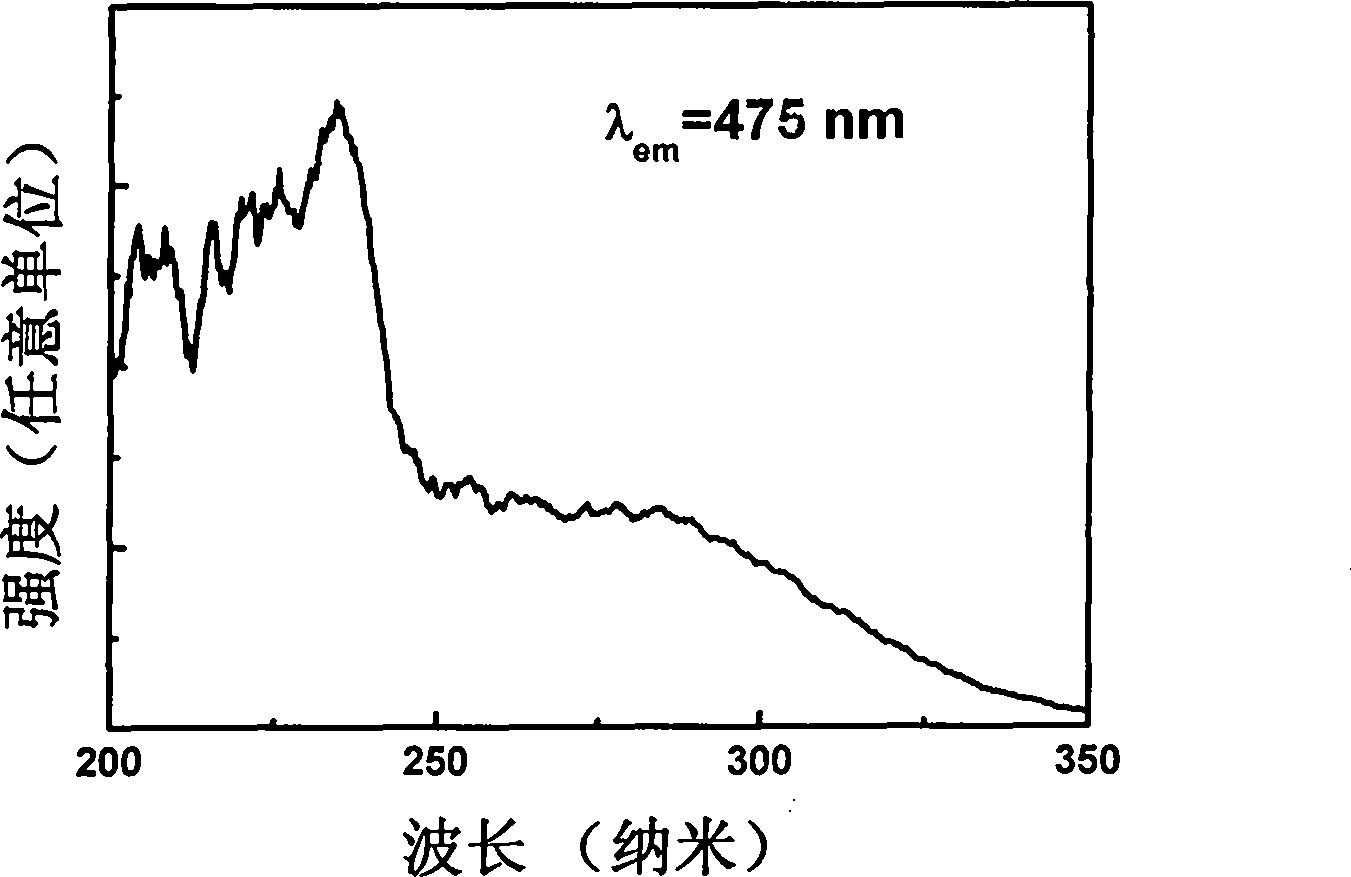
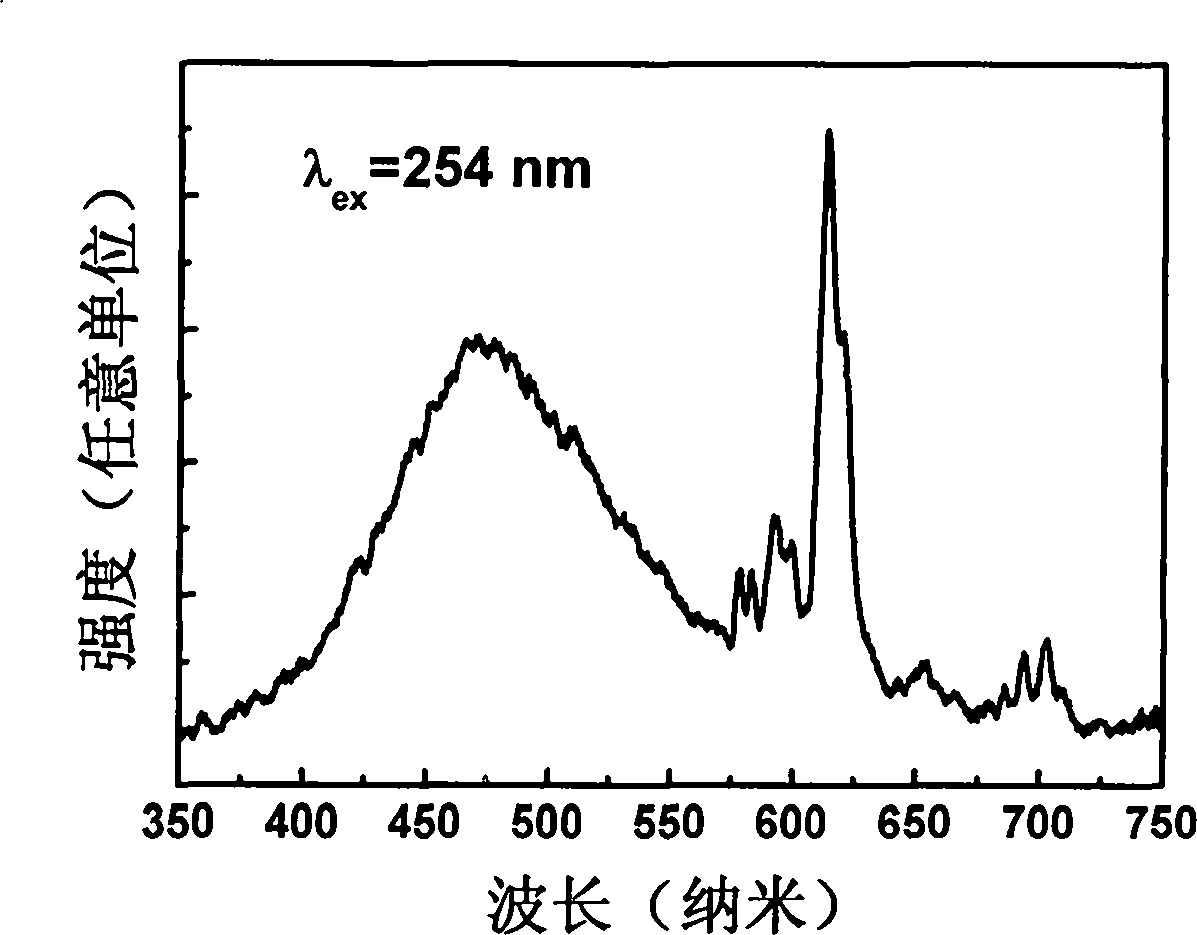
Rare-earth europium-activated single-component white-light fluorescent powder and preparation method thereof
A fluorescent powder and single-component technology, which is applied in the direction of chemical instruments and methods, luminescent materials, and the use of gas discharge lamps, etc., can solve the problems of complex preparation process, complex chemical composition, and complex preparation process, and achieve low preparation cost. Wide luminous color gamut, simple and easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh raw material CaCO 3 9.828g, Eu 2 o 3 0.352g, ZrO 2 12.347g, of which CaCO 3 and ZrO 2 Analytical pure, Eu 2 o 3 The purity is 99.9%. Put the raw materials into an agate mortar, add an appropriate amount of absolute ethanol and grind them thoroughly, then put them into a corundum crucible, place them in a heating furnace, and carry out initial burning in an atmospheric environment. The burning temperature is 1300°C, and the reaction time is 3 hours, and cooled to room temperature naturally after the reaction. The product obtained in the first step was mixed with 23.052g NH 4 h 2 PO 4 (Analytical pure) was put into an agate mortar, added an appropriate amount of absolute ethanol to grind and mix well, then put into a corundum crucible, and carried out secondary firing in an atmospheric environment, the firing temperature was 1200°C, and the reaction time was 6 hours. After the reaction was completed, the sample was taken out after being naturally cooled t...
Embodiment 2
[0026] Weigh raw material CaCO 3 9.728g, Eu 2 o 30.528g, ZrO 2 12.347g, of which CaCO 3 and ZrO 2 Analytical pure, Eu 2 o 3 The purity is 99.9%. Put the raw materials into an agate mortar, add an appropriate amount of absolute ethanol and grind them thoroughly, then put them into a corundum crucible, place them in a heating furnace, and carry out initial burning in an atmospheric environment. The burning temperature is 1250°C, and the reaction time is 2 hours, and cooled to room temperature naturally after the reaction. The product obtained in the first step was mixed with 23.052g NH 4 h 2 PO 4 (analytical pure) was put into an agate mortar, added an appropriate amount of absolute ethanol to grind and mix evenly, then put into a corundum crucible, and carried out secondary firing in an atmospheric environment, the firing temperature was 1100°C, and the reaction time was 10 hours. After the reaction was completed, the sample was taken out after being naturally cooled...
Embodiment 3
[0028] Weigh raw material CaCO 3 9.628g, Eu 2 o 3 0.705g, ZrO 2 12.347g, of which CaCO 3 and ZrO 2 Analytical pure, Eu 2 o 3 The purity is 99.9%. Put the raw materials into an agate mortar, add an appropriate amount of absolute ethanol and grind them thoroughly, then put them into a corundum crucible, place them in a heating furnace, and perform initial burning in an atmospheric environment. The burning temperature is 1200°C and the reaction time is 4 hours, and cooled to room temperature naturally after the reaction. The product that the first step makes and 23.052gNH 4 h 2 PO 4 (analytical pure) was put into an agate mortar, added an appropriate amount of absolute ethanol to grind and mix evenly, then put it into a corundum crucible, and carried out secondary firing in an atmospheric environment, the firing temperature was 1100°C, and the reaction time was 24 hours. After the reaction was completed, the sample was taken out after being naturally cooled to room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com