Preparation method of thermo-sensitive drug sustained and controlled release mesoporous composite
A composite material, temperature-sensitive technology, applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of complex synthesis process, achieve high drug loading, The effect of controlled release improvement and structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis experiment: the mesoporous SiO 2 The nanoparticles were activated at 120°C for 5 hours, placed in a desiccator and cooled to room temperature naturally, and then sealed and stored. Dissolve 0.15g of N-isopropylacrylamide, 0.0024g of N,N'-methylenebisacrylamide, 0.0033g of azobisisobutyronitrile in 3mL of acetone to form a solution, and take 0.15g of activated mesoporous SiO 2 Nanoparticles were added thereinto, and finally heated at 60° C. for 12 h under the protection of nitrogen, cooled to room temperature, filtered, washed, and vacuum-dried to obtain a temperature-sensitive mesoporous composite material.
[0028] Drug adsorption: Dissolve 1.2g of the slightly soluble drug ibuprofen in 30mL of n-hexane, add 0.3g of the synthesized mesoporous composite material into the above solution, stir at 45°C for 48h, filter, wash, and filter the solid Vacuum dried to obtain the drug-loaded sample.
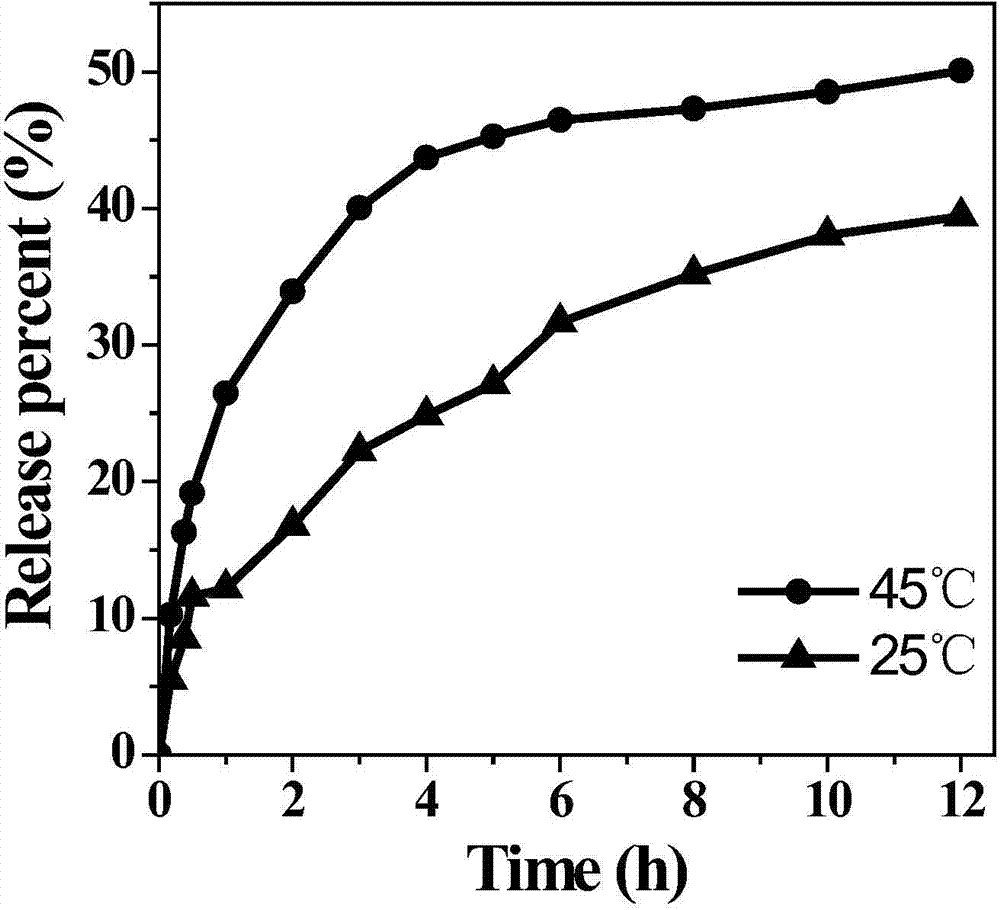
[0029] Drug sustained and controlled release experiment: Take 0.05g ...
Embodiment 2
[0033] Synthesis experiment: the mesoporous SiO 2 The nanoparticles were activated at 120°C for 5 hours, placed in a desiccator and cooled to room temperature naturally, and then sealed and stored. Dissolve 0.15g of N-isopropylacrylamide, 0.0024g of N,N'-methylenebisacrylamide, 0.0017g of azobisisobutyronitrile in 3mL of acetone to form a solution, and take 0.15g of activated mesoporous SiO 2 Nanoparticles were added thereinto, and finally heated at 60° C. for 12 h under the protection of nitrogen, cooled to room temperature, filtered, washed, and vacuum-dried to obtain a temperature-sensitive mesoporous composite material.
[0034] Drug adsorption: Dissolve 1.2g of the slightly soluble drug ibuprofen in 30mL of n-hexane, add 0.3g of the synthesized mesoporous composite material into the above solution, stir at 45°C for 48h, filter, wash, and filter the solid Vacuum dried to obtain the drug-loaded sample.
[0035] The drug-carrying system carries out the sensitive release of...
Embodiment 3
[0037] Synthesis experiment: the mesoporous SiO 2 The nanoparticles were activated at 120°C for 5 hours, placed in a desiccator and cooled to room temperature naturally, and then sealed and stored. Dissolve 0.15g of N-isopropylacrylamide, 0.0048g of N,N’-methylenebisacrylamide, 0.0033g of azobisisobutyronitrile in 3mL of acetone to form a solution, and take 0.15g of activated mesoporous SiO 2 Nanoparticles were added thereinto, and finally heated at 60° C. for 12 h under the protection of nitrogen, cooled to room temperature, filtered, washed, and vacuum-dried to obtain a temperature-sensitive mesoporous composite material.
[0038] Drug adsorption: Dissolve 1.2g of the slightly soluble drug ibuprofen in 30mL of n-hexane, add 0.3g of the synthesized mesoporous composite material into the above solution, stir at 45°C for 48h, filter, wash, and filter the solid Vacuum dried to obtain the drug-loaded sample.
[0039] The drug-carrying system carries out the sensitive release of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesoporous | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com