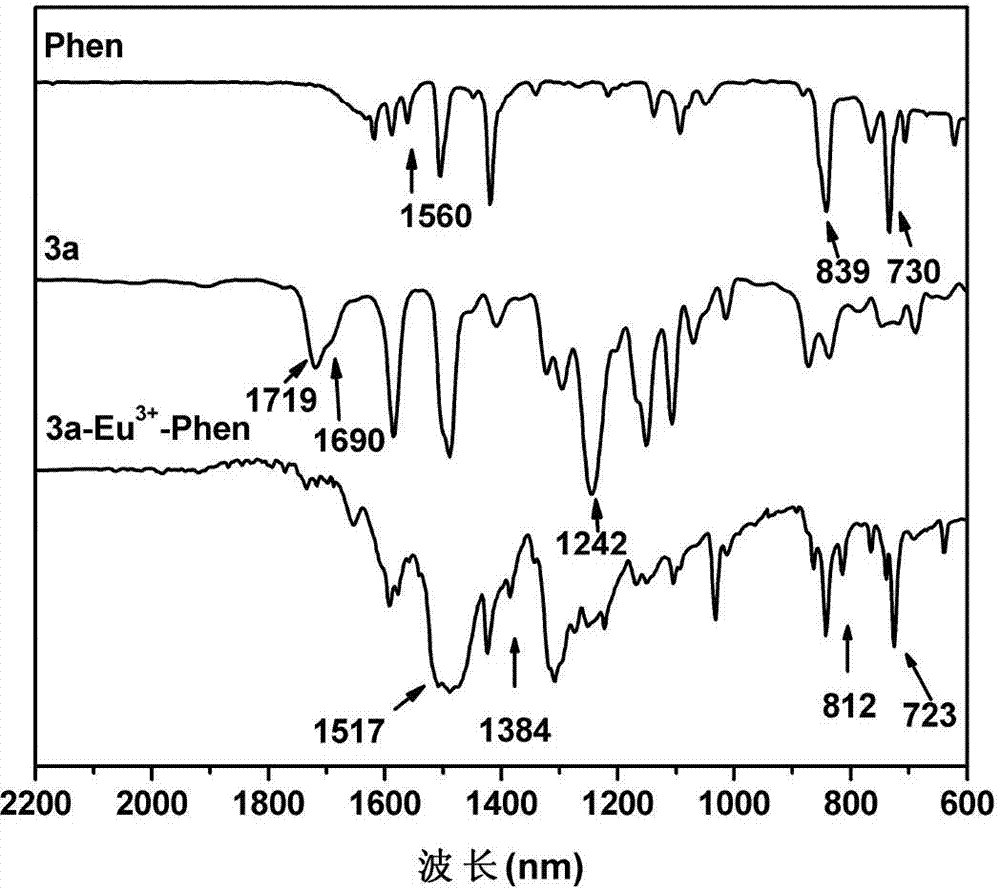
Azo polyarylether rare earth complex material as well as preparation method and application thereof
A technology of rare earth complexes and polyarylethers, applied in the direction of luminescent materials, chemical instruments and methods, instruments, etc., can solve the problems of reducing material performance, agglomeration, etc., and achieve the effects of improving dispersion, increasing concentration, and reducing impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
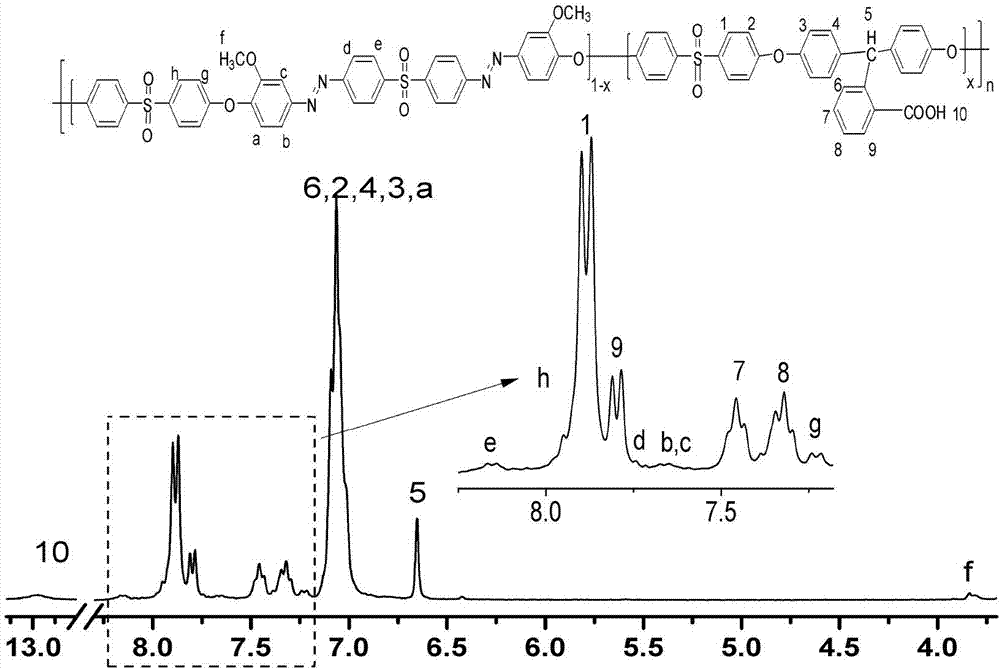
[0046] 0.5185g (0.001mol) 3,3'-dimethoxy-4,4'-dihydroxyphenyl azodiphenyl sulfone, 2.8331 (0.009mol) 4,4'-dihydroxytriphenylmethane-2 -Carboxylic acid and 2.5425g (0.01mol) 4,4'-difluorodiphenyl sulfone were added to the three-necked flask, and 2.3492g (0.017mol) K 2 CO 3 , 12ml of toluene and 19ml of dimethyl sulfoxide (DMSO), stirred and heated to reflux 120-130°C under the protection of nitrogen, removed the water in the system, then released the toluene in the system, raised the temperature to 170°C, and reacted for another 4 hours. The reaction solution was poured into an aqueous hydrochloric acid solution. After the precipitated polymer was pulverized, it was washed with water and ethanol several times to remove organic solvents and inorganic salts, and dried under vacuum at 80°C to obtain an orange-red polymer with a yield of 93% and a number average molecular weight (Mn) of 1.6×10 4 , The viscosity ηiv measured by Ubbelohde viscometer is 0.39 (dL / g), indicating that ...
Embodiment 2
[0049] 1.037g (0.002mol) 3,3'-dimethoxy-4,4'-dihydroxyphenyl azodiphenylsulfone, 2.5627g (0.008mol) 4,4'-dihydroxytriphenylmethane- 2-Carboxylic acid and 2.5425g (0.01mol) 4,4'-difluorodiphenyl sulfone were added to the three-necked flask, and 2.211g (0.016mol) K 2 CO 3 , 12ml of toluene and 19ml of dimethyl sulfoxide (DMSO), stirred and heated to reflux 120-130°C under the protection of nitrogen, removed the water in the system, then released the toluene in the system, raised the temperature to 170°C, and reacted for another 4 hours. The reaction solution was poured into an aqueous hydrochloric acid solution. After the precipitated polymer was pulverized, it was washed with water and ethanol several times to remove organic solvents and inorganic salts, and dried in vacuum at 80°C to obtain an orange-red polymer with a yield of 90%. The viscosity ηiv measured by Ubbelohde viscometer was 0.38 (dL / g). Its structural formula is shown in A-3b.
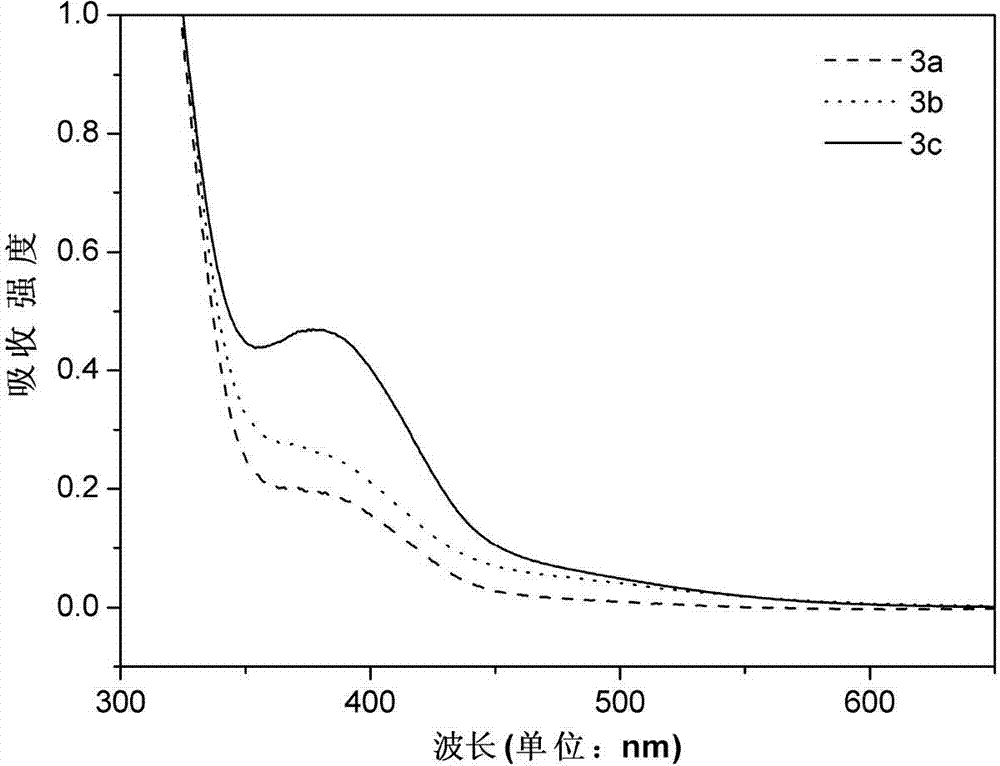
[0050] figure 2 The ultraviol...
Embodiment 3
[0052] 1.5555g (0.003mol) 3,3'-dimethoxy-4,4'-dihydroxyphenyl azodiphenyl sulfone, 2.2424g (0.007mol) 4,4'-dihydroxytriphenylmethane- 2-Carboxylic acid and 2.5425g (0.01mol) 4,4'-difluorodiphenyl sulfone were added to the three-necked flask, and 2.0729g (0.015mol) K 2 CO 3 , 12ml of toluene and 19ml of dimethyl sulfoxide (DMSO), stirred and heated to reflux 120-130°C under the protection of nitrogen, removed the water in the system, then released the toluene in the system, raised the temperature to 170°C, and reacted for another 4 hours. The reaction solution was poured into an aqueous hydrochloric acid solution. After the precipitated polymer was pulverized, it was washed with water and ethanol several times to remove organic solvents and inorganic salts, and dried under vacuum at 80°C to obtain an orange-red polymer with a yield of 87%. The viscosity ηiv measured by Ubbelohde viscometer was 0.36 (dL / g). Its structural formula is shown in A-3c.
[0053] figure 2 The ult...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com