Method for detecting cancer cell using fluorescently labeled L-glucose derivative, and cancer cell-imaging agent comprising fluorescently labeled L-glucose derivative
A technology of a glucose derivative and a detection method, which is applied in the field of detecting cancer cells or cells that may have cancer, and can solve the problems of difficulty in diagnosing diabetes patients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1: the synthesis of compound
[0112] (1) Synthesis of fluorescently labeled L-glucose derivatives
[0113] (1-1) Synthesis of 2-NBDLG
[0114] 2-NBDLG is synthesized from L-glucose as follows.
[0115]
[0116] 3,4,6-Tri-O-acetyl-L-glucal
[0117] L-glucose (20 g) was dissolved in pyridine (262 ml), and cooled to 0°C. Add anhydrous acetic acid (171ml) dropwise, and stir overnight at room temperature. Concentration under reduced pressure followed by azeotropic operation with toluene was repeated three times. Ethyl acetate was added to the residue, washed with saturated sodium bicarbonate water and saturated brine, and the organic layer was dried over sodium sulfate. Sodium sulfate was filtered off, and the organic solvent was removed by concentration under reduced pressure. The obtained residue was dissolved in dehydrated dichloromethane (115 ml) under an argon atmosphere, and cooled to 0°C. A 30% hydrobromide acetic acid solution (61 ml) was adde...
reference example 1
[0211] Reference Example 1: Synthesis of Comparative Compounds
[0212] (1) Synthesis of fluorescently labeled D-glucose derivatives
[0213] (1-1) Synthesis of 2-NBDG
[0214] 2-NBDG can be synthesized according to the method described in Non-Patent Document 5.
[0215] (1-2) Synthesis of fluorescently labeled L-mannose
[0216] In the present invention, as the sugar skeleton bound to the fluorescent group, the mirror-image isomer of the natural 6-carbon sugar D-glucose that exists in large quantities in nature can be used, that is, the non-natural L-glucose that is not found in nature. On the other hand, as a fluorescently labeled L-mannose derivative whose backbone is the mirror image isomer of the 6-carbon sugar D-mannose that exists in large quantities in nature, that is, unnatural L-mannose, the inventors newly synthesized 2-Deoxy-2-[N-(7-nitrobenzo-2-oxa-1,3-oxadiazol-4-yl)amino]-L-mannose (2-NBDLM).
[0217]
[0218] Benzyl 4,6-O-benzylidene-α-L-glucopyranosid...
Embodiment 2
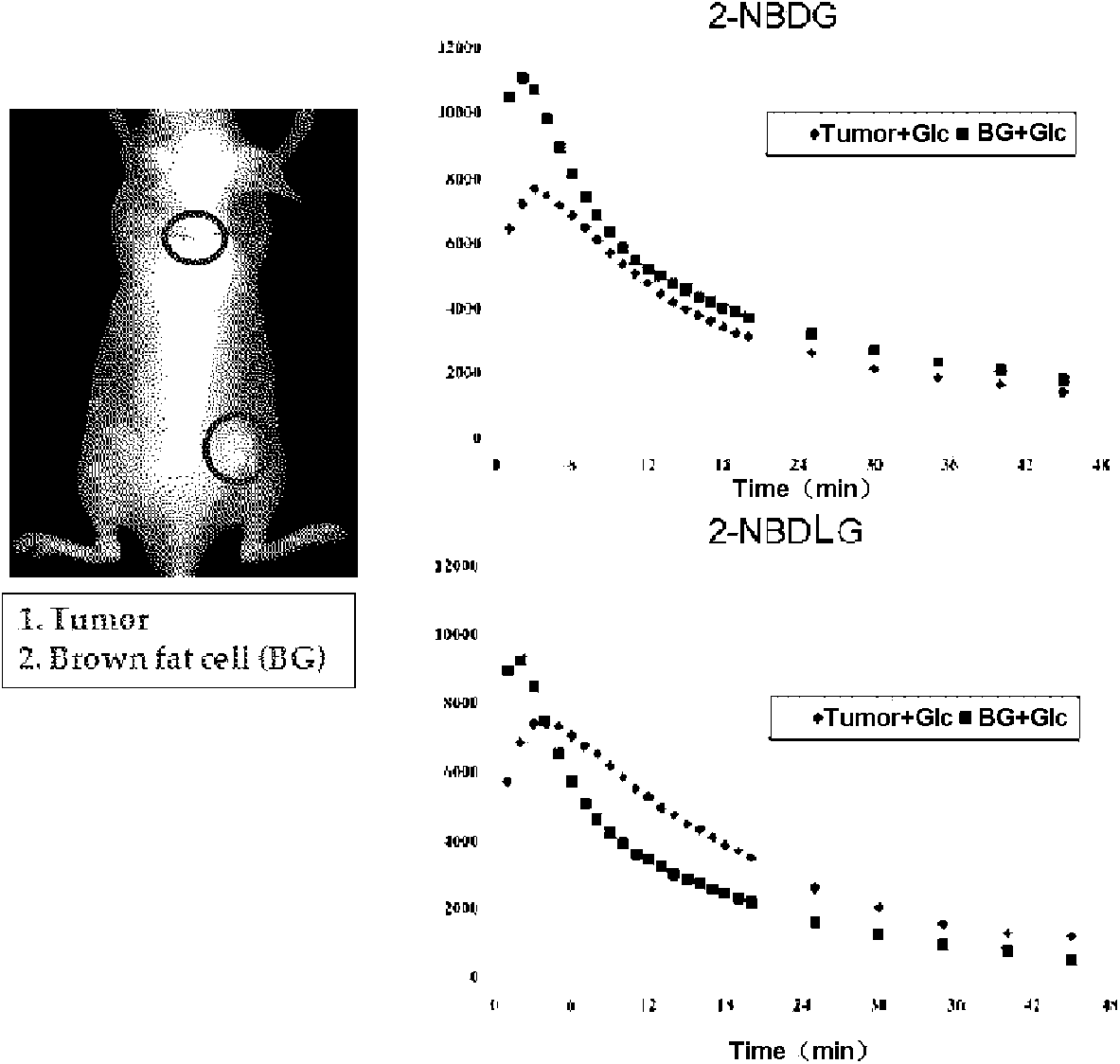
[0239] Example 2: Studies Using Mice Transplanted with Tumor Cells (C6 Glioma)
[0240] (experimental method)
[0241] (1) Preparation of rat glioma cells (C6) for transplantation
[0242] According to the conventional method, the number of cells was 2×10 7 cells / mL to prepare C6 cells.
[0243] (1-1) Cultivation of C6 cells
[0244] Transplant the C6 cells into a 10cm culture dish in CO 2 Let stand in the incubator and cultivate at 37°C. The culture medium was changed every 2 days.
[0245] (1-2) Composition of culture medium for culturing C6 cells
[0246] Add Fetal Bovine Serum (Equitech-Bio SFBM) to Dulbecco's modified Eagle's Medium (DMEM) (Nacalai No.08456-65) containing 1g / L glucose to make the final concentration 10%, and add penicillin-chain Mycin (Nacalai No. 26253-84) was used at a final concentration of 1%, and the above culture solution was used.
[0247] (2) Production of tumor model mice
[0248] For 5-6 week-old thymus-deficient nude mice (BALB / cAJcl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com