A method for comprehensive utilization of potassium, boron and lithium in carbonate-type salt lake brine
A carbonate type, salt lake brine technology, applied in lithium carbonate;/acid carbonate, boron oxide, alkali metal halide purification and other directions, can solve the problem of comprehensive utilization and reduce lithium extraction efficiency , limit the large-scale extraction of lithium, etc., to achieve the effect of good product quality, simple process and high resource utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
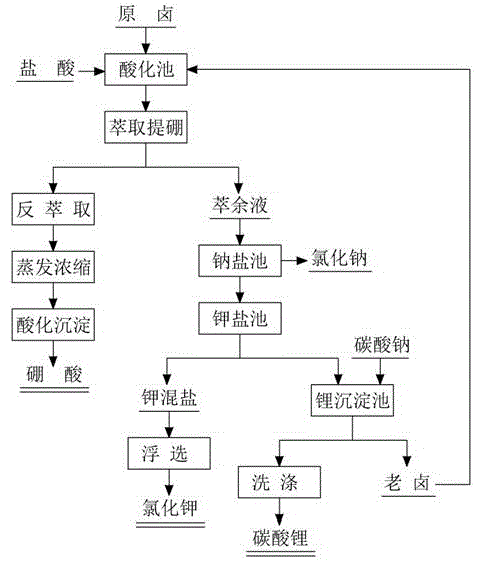
[0026] A carbonate-type salt lake brine, its main composition is K2.93%, Na9.82%, CO 3 2- 2.27%, B 2 o 3 0.66%, Li0.07%.
[0027] (1) In the acidification pool, add hydrochloric acid to the original brine to cause a neutralization reaction, and adjust the pH of the brine to 2.0;
[0028] (2) Extract the acidified brine. Under the extraction conditions of 2-ethyl-1,3-ethanediol concentration of 15% and isooctyl alcohol concentration of 35%, the extraction rate is 96.32%. Under the condition of 0.625mol / L, the stripping rate is 98.50%. The stripping liquid is evaporated and concentrated 10 times, and boric acid is precipitated by acidification. After filtering, washing and drying, the boric acid product is obtained. The yield of boric acid is 90.90%, and the recovery rate of boron is 86.24%;
[0029] (3) The raffinate is introduced into the sodium salt pool, and concentrated by solar evaporation to a density of 1.296g / cm 3 , to precipitate sodium chloride-based sodium salt...
Embodiment 2
[0035] A carbonate-type salt lake brine, its main composition is K2.93%, Na9.82%, CO 3 2- 2.27%, B 2 o 3 0.66%, Li0.07%.
[0036] (1) In the acidification pool, add hydrochloric acid to the original brine to cause a neutralization reaction, and adjust the pH of the brine to 4.6;
[0037] (2) Extract the acidified brine. Under the extraction condition of 2-ethyl-1,3-ethanediol concentration of 50%, the extraction rate is 97.27%. Under the condition of NaOH concentration of 0.50mol / L , the stripping rate was 97.54%, the stripping solution was evaporated and concentrated by 8.2 times, and boric acid was precipitated by acidification, and the boric acid product was obtained after filtration, washing and drying. The yield of boric acid was 87.92%, and the boron recovery rate was 83.41%;
[0038] (3) The raffinate is introduced into the sodium salt pool, and concentrated by solar evaporation to a density of 1.309g / cm 3 , to precipitate sodium salt mainly composed of sodium chlo...
Embodiment 3
[0044] A carbonate-type salt lake brine, its main composition is K2.93%, Na9.82%, CO 3 2- 2.27%, B 2 o 3 0.66%, Li0.07%.
[0045] (1) In the acidification pool, add hydrochloric acid to the original brine, a neutralization reaction occurs, and the brine pH value is adjusted to 5.4;
[0046] (2) Extract the acidified brine. Under the extraction condition of 2-ethyl-1,3-ethanediol concentration of 30%, the extraction rate is 98.32%. Under the condition of NaOH concentration of 1.0mol / L , the stripping rate is 99.50%, the stripping solution is evaporated and concentrated 13 times, acidified and precipitated to produce boric acid, and the boric acid product is obtained by filtering, washing and drying, and the recovery rate of boron is 83.1%;
[0047] (3) The raffinate is introduced into the sodium salt pool, evaporated by the sun and concentrated to a density of 1.310g / cm 3 , to precipitate sodium chloride-based sodium salt;
[0048] (4) The mother liquor after the sodium s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com