Microporous aluminum-salt lithium adsorbent, preparation method therefor, filler and lithium ion enriching method
A lithium adsorption and lithium ion technology, which is applied in chemical instruments and methods, other chemical processes, alkali metal carbonates, etc., can solve the problem that the specific surface area and pore size of the adsorbent cannot meet the demand, the adsorption performance of the aluminum salt lithium adsorbent decreases, The advantages of the adsorption capacity of the adsorbent are not obvious, etc., to achieve the effects of concentration and enrichment and subsequent utilization processing, obvious selectivity advantages, and long cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] At normal temperature, 3mol / L lithium hydroxide solution was added dropwise to 1mol / L aluminum chloride solution at a rate of 200mL / h, and the ratio of the two in the system was 3:1, and the stirring speed during the reaction was 500rpm, control the pH of the reaction end point to stop dripping at 6 o'clock, and continue to stir for 30 minutes to stop the experiment, after aging for 24 hours, the solid-liquid separation, the liquid continues to be used for the synthesis of subsequent adsorbents, and after the solid is re-slurried for 3 times, the re-slurry Pulp washing is well-known to those skilled in the art after beating with water; then drying at 50°C, grinding and sieving, and the obtained adsorbent LiCl 2Al(OH) 3 ·H 2 The average pore diameter of O is 1.7 nm. figure 1 and figure 2 It is the SEM image of the microporous aluminum salt lithium adsorbent and the adsorbent and LiCl 2Al(OH) 3 ·H 2 Standard XRD comparison chart of O.
[0046] The adsorbent is mixed...
Embodiment 2
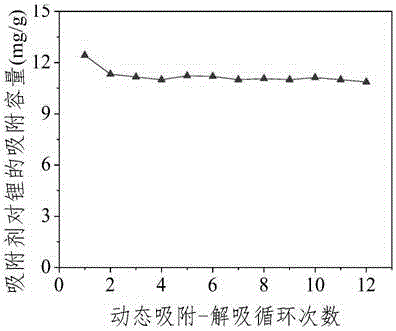
[0048] At room temperature, add 2mol / L lithium hydroxide solution dropwise to 2mol / L aluminum sulfate solution at a rate of 200mL / h, so that the ratio of the two in the system is 3:1.02, and the stirring speed during the reaction is 800rpm , control the pH of the reaction end point to stop dropping at 7 o'clock, and continue to stir for 30 minutes before stopping the experiment. After aging for 24 hours, the solid-liquid separation, the liquid continues to be used for the synthesis of subsequent adsorbents, and the solid is re-slurried and washed for 3 times, and then dried at 50 ° C. , powdered and sieved, the obtained adsorbent LiCl·2Al(OH) 3 1.1H 2 The average pore diameter of O is 2 nanometers, and the adsorbent is mixed with the binder and compressed into tablets for granulation. After crushing and sieving, the adsorbent with a particle size of 0.9mm≤Dp≤2mm was packed into a column for dynamic adsorption experiments (length-to-diameter ratio: 1.82) for adsorption experim...
Embodiment 3
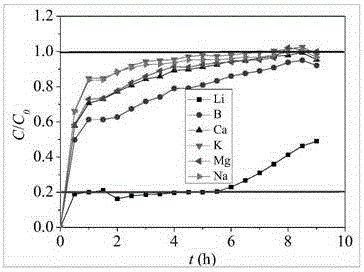
[0050] At room temperature, drop 3mol / L lithium hydroxide solution into 1.02mol / L aluminum nitrate solution at a rate of 200mL / h, and make the ratio of the two in the system 3:1.03, and the stirring speed during the reaction is 700rpm, control the pH of the reaction end point to stop dropping at 6 o'clock, and continue to stir for 30 minutes before stopping the experiment. After aging for 24 hours, the solid and liquid are separated, and the liquid continues to be used for the synthesis of subsequent adsorbents. dry, powdered and sieved, the obtained adsorbent LiCl 2Al(OH) 3 0.9H 2 The pore diameter of O is 1.8 nanometers, and the adsorbent is mixed with the binder and compressed into tablets for granulation. After crushing and sieving, the adsorbent with a particle size of 0.9mm≤Dp≤2mm was packed into a column for dynamic adsorption experiments (length-to-diameter ratio: 2.16) for adsorption experiments. The concentrated brine containing lithium 3g / L of the adsorbent is use...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com