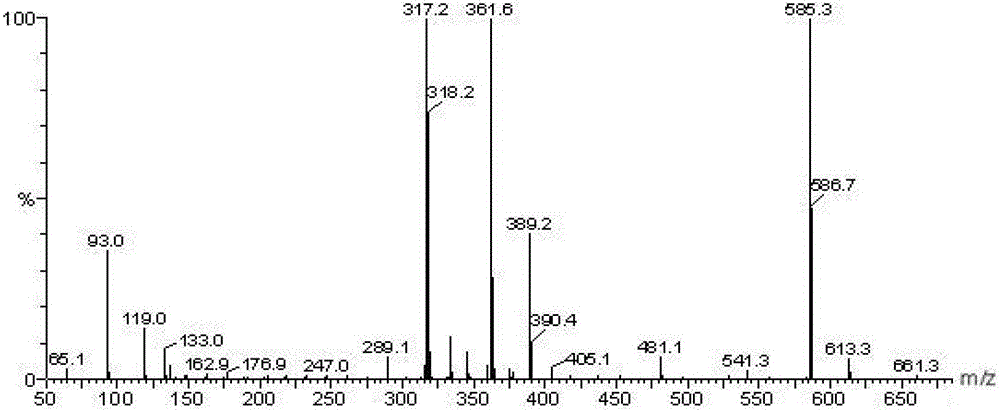
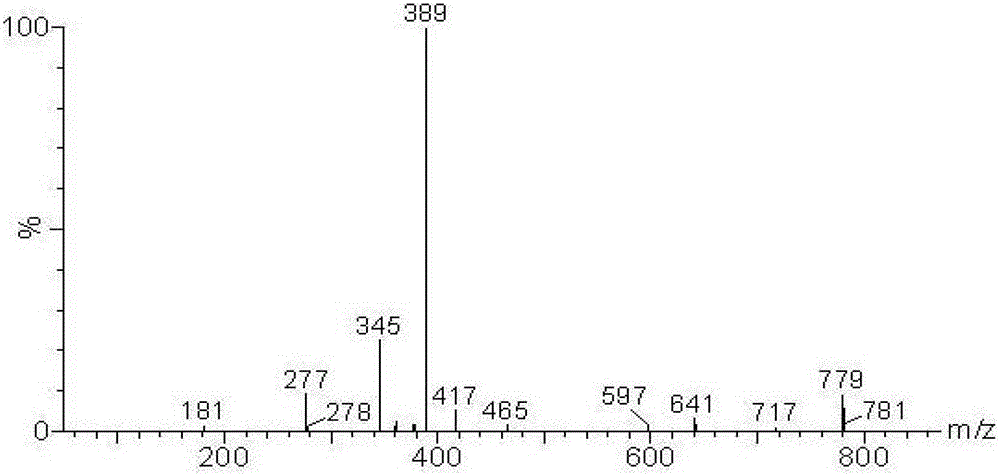
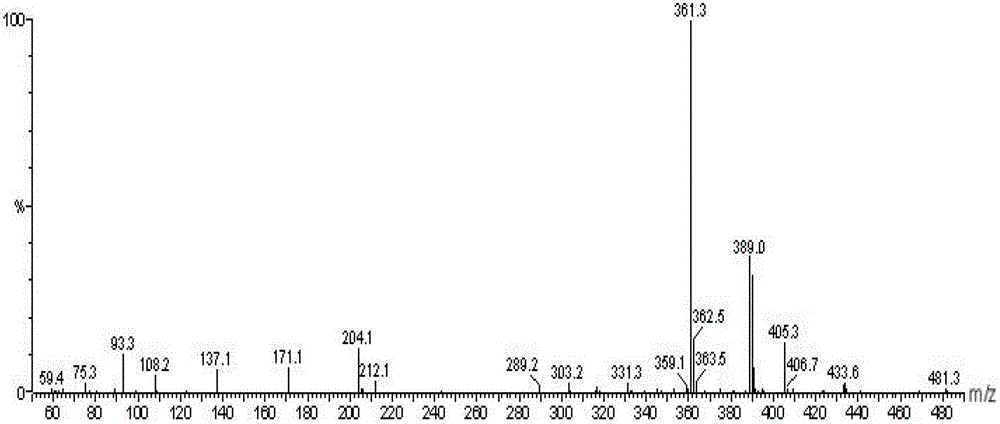
Alkyl salicylic acid synthesis method
A technology for the synthesis of alkyl salicylic acid and its method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., which can solve problems such as inability to recycle, high equipment requirements, and reduced utilization rate of raw material olefins, etc. To achieve the effect of environmental protection and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 112.2 g of 1-hexadecene was added to a 500 ml four-necked round-bottom flask, stirred with a magnetic stirrer, 69.0 g of salicylic acid was slowly added, and then 13.8 g of benzenesulfonic acid was added. Nitrogen gas was introduced, the reaction temperature was 120° C., and the reaction time was 7 hours. After the reaction is finished, water is added to wash to remove the catalyst, and after standing to separate layers to remove water, the product alkyl salicylic acid is obtained. The conversion rate of olefin reaction was 89.6%.
Embodiment 2
[0025] 126.2 g of 1-octadecene was added to a 500 ml four-neck round-bottom flask, stirred with a magnetic stirrer, 69.0 g of salicylic acid was slowly added, and then 13.8 g of benzenesulfonic acid was added. Nitrogen gas was introduced, the reaction temperature was 130° C., and the reaction time was 7 hours. After the reaction is finished, water is added to wash to remove the catalyst, and after standing to separate layers to remove water, the product alkyl salicylic acid is obtained. The conversion rate of olefin reaction was 76.2%.
Embodiment 3
[0027] Add 117.6 g of C16C18 alkenes to a 500 ml four-neck round bottom flask, stir with a magnetic stirrer, slowly add 69.0 g of salicylic acid, and then add 13.8 g of benzenesulfonic acid. Nitrogen gas was introduced, the reaction temperature was 130° C., and the reaction time was 7 hours. After the reaction is finished, water is added to wash to remove the catalyst, and after standing to separate layers to remove water, the product alkyl salicylic acid is obtained. The conversion rate of olefin reaction was 83.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com