Components of catalyst used for olefin polymerization and preparation method thereof
A technology for olefin polymerization and catalyst, which is applied in the field of components for olefin polymerization catalyst and its preparation, and can solve the problems such as molecular weight distribution and hydrogen modulation sensitivity that need to be investigated and are not satisfactory.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
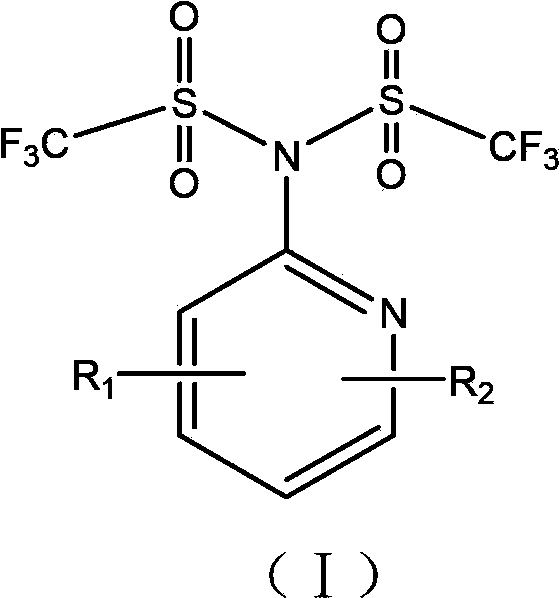
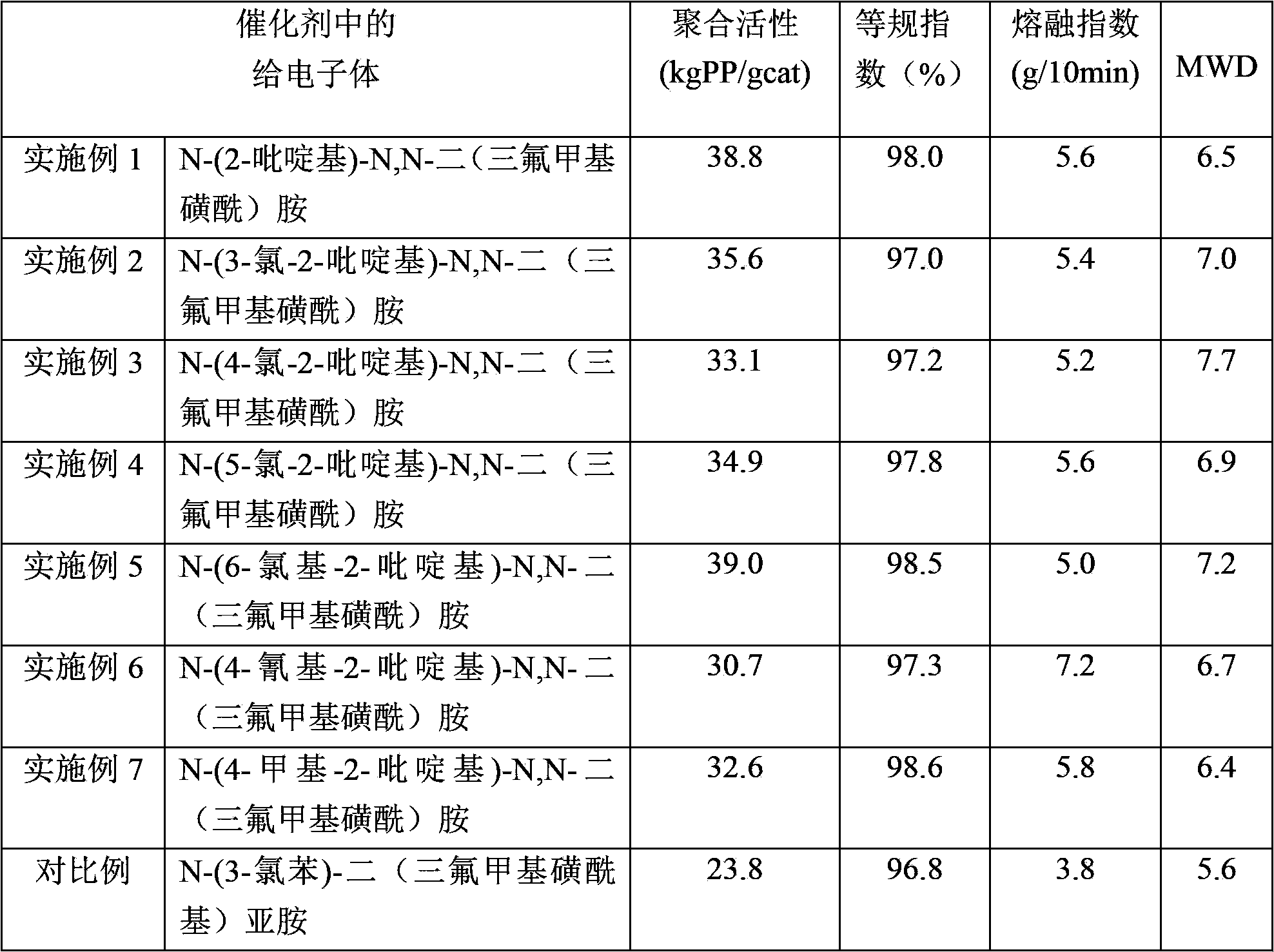
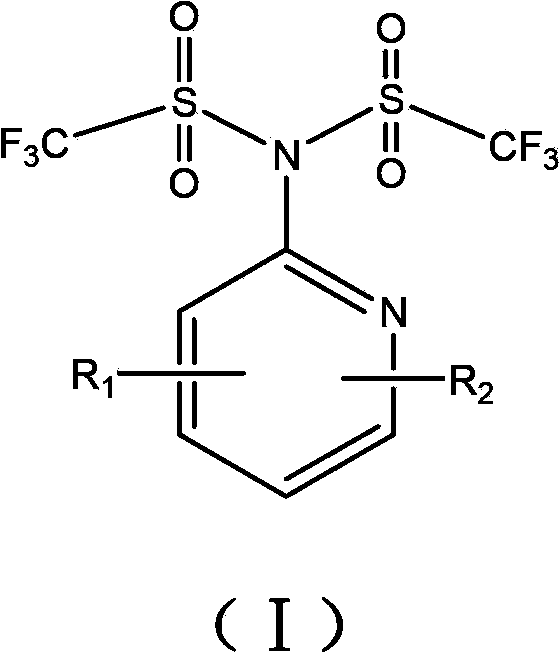
[0063] Example 1 Synthesis of compound N-(2-pyridyl)-N,N-bis(trifluoromethylsulfonyl)amine
[0064] In a 500mL reaction flask, after nitrogen purging, 2-aminopyridine (4.7g, 0.05mol), pyridine (9.7mL, 0.12mol) and dichloroethane (150mL) were added in the presence of nitrogen, and the mixture was stirred After cooling to -78°C, a mixture of trifluoromethanesulfonic anhydride (20.2 mL, 0.12 mol) and dichloroethane (50 mL) was added dropwise, and stirred at this temperature for 2 h after the addition was complete. Then gradually warm up to room temperature, react at room temperature for 20 h, and add 50 mL of water to terminate the reaction. The aqueous phase was separated and extracted twice with dichloroethane. The organic phases were combined and washed with 10% aqueous sodium hydroxide solution and saturated brine respectively. The organic phase was dried over anhydrous sodium sulfate and the solvent was removed. Column chromatography, using a mixture of petroleum ether: eth...
Embodiment 2
[0066] Example 2 Synthesis of compound N-(3-chloro-2-pyridyl)-N,N-bis(trifluoromethylsulfonyl)amine
[0067] In a 500 mL reaction flask, after nitrogen purge, add 2-amino-5-chloropyridine (6.4 g, 0.05 mol), pyridine (9.7 mL, 0.12 mol) and dichloroethane (150 mL) in the presence of nitrogen , the mixture was cooled to -78°C with stirring, and a mixture of trifluoromethanesulfonic anhydride (20.2 mL, 0.12 mol) and dichloroethane (50 mL) was added dropwise, and stirred at this temperature for 2 h after the addition was complete. Then gradually warm up to room temperature, react at room temperature for 20 h, and add 50 mL of water to terminate the reaction. The aqueous phase was separated and extracted twice with dichloroethane. The organic phases were combined and washed with 10% aqueous sodium hydroxide solution and saturated brine respectively. The organic phase was dried over anhydrous sodium sulfate and the solvent was removed. Column chromatography, using a mixture of petro...
Embodiment 3
[0069] Example 3 Synthesis of compound N-(4-chloro-2-pyridyl)-N,N-bis(trifluoromethylsulfonyl)amine
[0070] In a 500 mL reaction flask, after nitrogen purge, add 2-amino-4-chloropyridine (6.4 g, 0.05 mol), pyridine (9.7 mL, 0.12 mol) and dichloroethane (150 mL) in the presence of nitrogen , the mixture was cooled to -78°C with stirring, and a mixture of trifluoromethanesulfonic anhydride (20.2 mL, 0.12 mol) and dichloroethane (50 mL) was added dropwise, and stirred at this temperature for 2 h after the addition was complete. Then gradually warm up to room temperature, react at room temperature for 20 h, and add 50 mL of water to terminate the reaction. The aqueous phase was separated and extracted twice with dichloroethane. The organic phases were combined and washed with 10% aqueous sodium hydroxide solution and saturated brine respectively. The organic phase was dried over anhydrous sodium sulfate and the solvent was removed. Column chromatography, using a mixture of petro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com