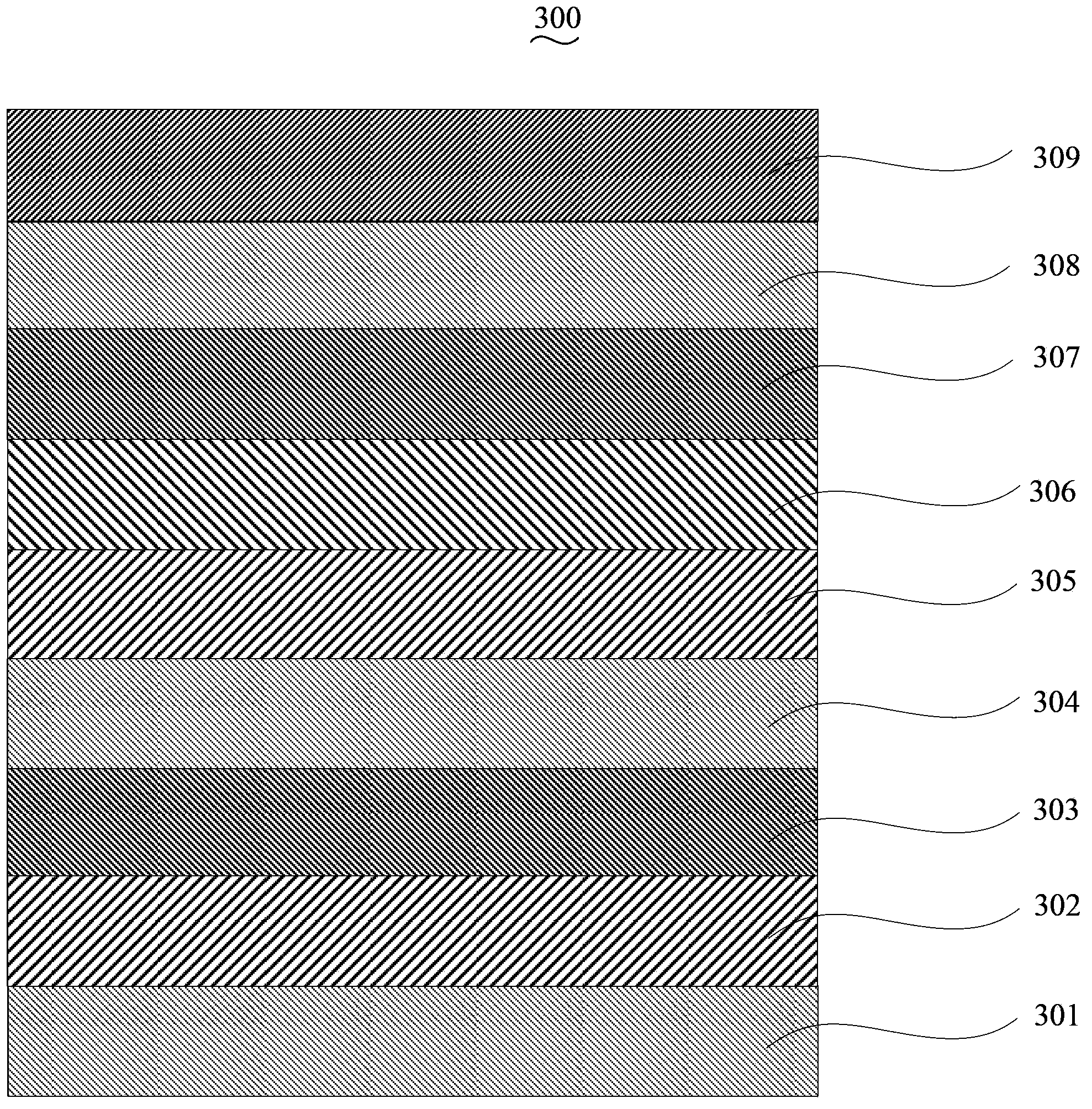
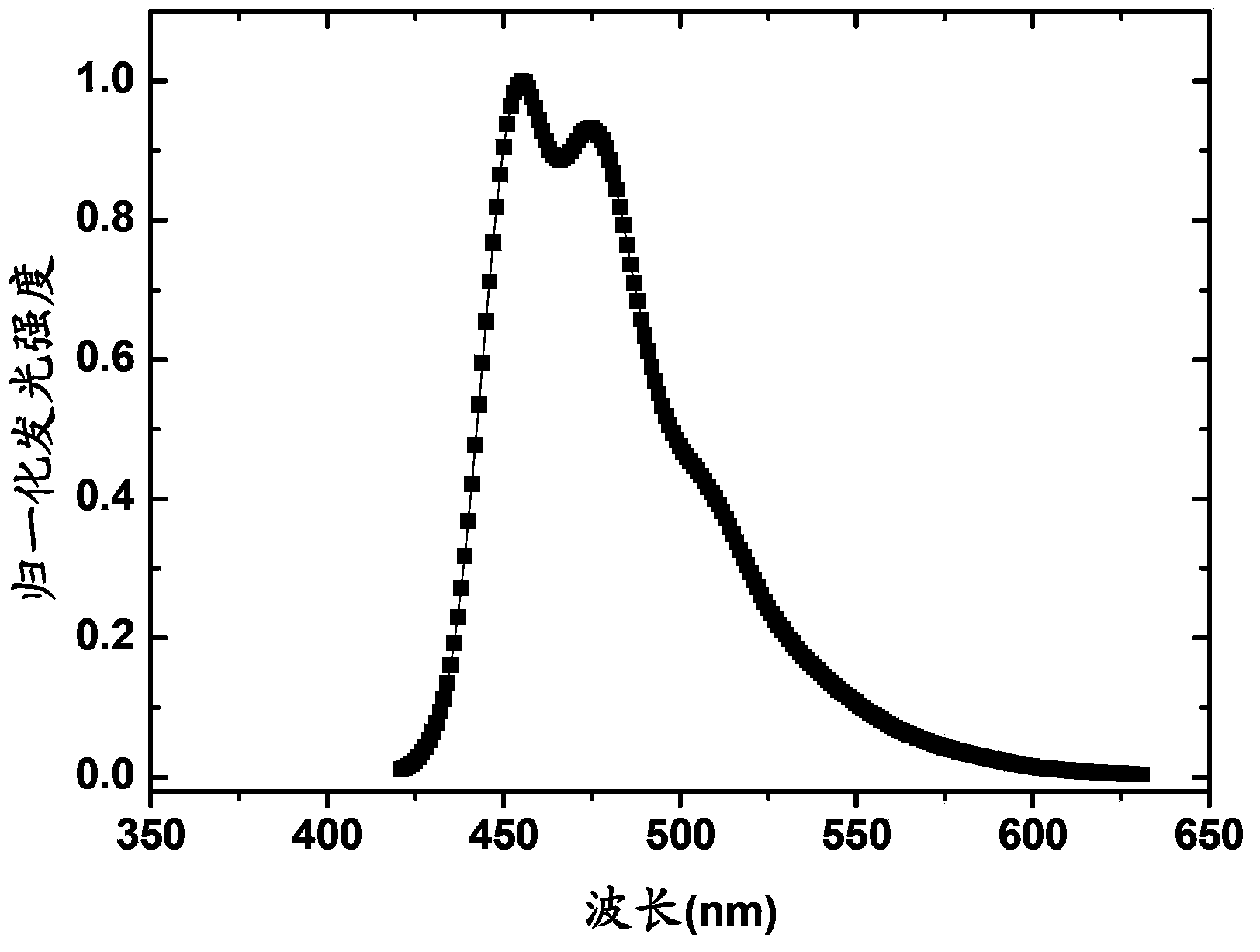
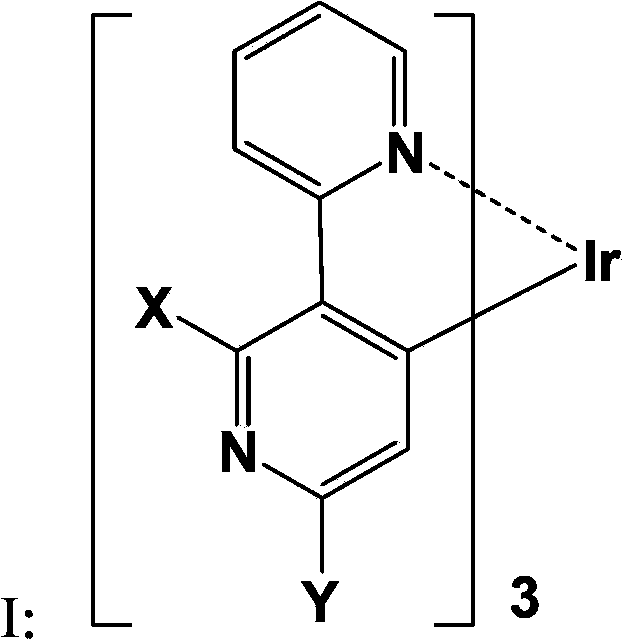
Iridium-containing organic electroluminescent material, its preparation method and organic electroluminescent device
A luminescent and electromechanical technology, applied in the fields of luminescent materials, electric solid devices, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of the above-mentioned iridium-containing organic electroluminescent material comprises the following steps:
[0040] The following steps are carried out under anhydrous and anaerobic conditions unless otherwise specified, such as in N 2 Or under an inert gas atmosphere, etc., the solvent used can also adopt other solvents that have better compatibility with the reactants except the solvents given in each step.
[0041] S1, providing compound C and compound D represented by the following structural formula,
[0042]
[0043] S2. Under anhydrous and oxygen-free conditions, compound C and compound D are carried out in an organic solvent in a Suzuki reaction in a molar ratio of 1:1 to 1:2 under the condition of a catalyst, and compound A is obtained after purification. The reaction formula is as follows:
[0044]
[0045] In this step, preferably, the catalyst used in the Suzuki reaction is a cocatalyst composed of an inorganic base and an org...
Embodiment 1
[0059] Example 1: Complex three (2',6'-dichloro-2,3'-bipyridine-N,C 2 ’) Synthesis of iridium
[0060] 1. Synthesis of 2,6-dichloro-3,2'-bipyridine
[0061] Under nitrogen protection, (0.95g, 6.00mmol) 2-bromopyridine, (1.38g, 7.20mmol) 2,6-dichloro-3-pyridineboronic acid, (3.04g, 22mmol) K 2 CO 3 and (0.35g,0.30mmol)Pd(PPh 3 ) 4 Dissolve in a mixed solution of 30mL toluene and 5mL water, heat to 100°C, and stir for 20h. After cooling to room temperature, the aqueous phase in the reaction mixture was separated and extracted with ethyl acetate (3×100 mL), and the organic phases were combined. The combined organic phases were washed with brine and dried over anhydrous magnesium sulfate. Filter and evaporate the solvent to obtain the crude product. The mixture of chloroform and n-hexane with a volume ratio of 1:3 was used as the eluent for silica gel column chromatography to obtain 0.78 g of a white solid with a yield of 57.8%. The reaction equation is as follows:
[006...
Embodiment 2
[0072] Example 2: Complex three (2',6'-difluoro-2,3'-bipyridine-N,C 2 ’) Synthesis of iridium
[0073] 1. For the synthesis steps of 2,6-difluoro-3,2'-bipyridine, see Example 1
[0074] Under nitrogen protection, (0.95g, 6.00mmol) 2-bromopyridine, (1.14g, 7.20mmol) 2,6-difluoro-3-pyridineboronic acid, (3.04g, 22mmol) K 2 CO 3 and (0.35g,0.30mmol)Pd(PPh 3 ) 4 Dissolve in a mixed solution of 30mL toluene and 5mL water, heat to 100°C, and stir for 20h. After cooling to room temperature, the aqueous phase in the reaction mixture was separated and extracted with ethyl acetate (3×100 mL), and the organic phases were combined. The combined organic phases were washed with brine and dried over anhydrous magnesium sulfate. Filter and evaporate the solvent to obtain the crude product. The mixture of chloroform and n-hexane with a volume ratio of 1:3 was used as the eluent for silica gel column chromatography to obtain 0.69 g of a white solid with a yield of 59.8%. The reaction eq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com