Double-target-spot fusion protein capable of enhancing TRAIL antitumor activity
A technology of anti-tumor activity and fusion protein, applied in] The present invention relates to the technical field of genetic engineering drugs, which can solve the problems of increasing the sensitivity of exogenous TRAIL, achieve high recovery and purification efficiency, strong anti-tumor effect, and enhance activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
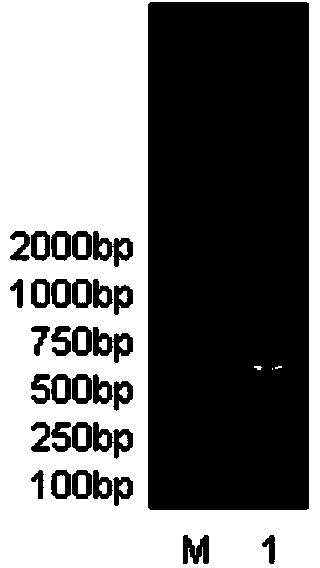
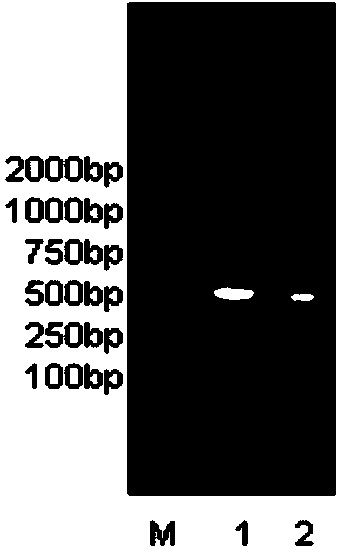
[0057] Splicing and synthesis of full-length fusion gene sequences 1 and 2
[0058] The target of TRAIL protein (114-281aa) in the designed fusion protein is TRAIL receptor DR 4 、DR 5 , SMAC N7 peptide is aimed at the apoptosis antagonist XIAP, in order to have a significant synergistic effect on TRAIL.
[0059] The full-length fusion gene sequence to be synthesized (see sequence 1, 4) is divided into two parts: the fusion front sequence (see sequence 3, 6) and the TRAIL sequence (see sequence 7). The fusion front sequence includes (R8+SMAC N7+AP) (see sequence 3) and (TAT+SMAC N7+AP) (see sequence 6) peptide coding cDNA sequences. The double-strand splicing primers (see sequence 8-18) were used as raw materials, and the synthesized double-strand splicing primers were reacted together. Under the condition of no Taq DNA Polymerase, the spliced fusion front sequence (R8+SMAC N7+AP) and (TAT +SMAC N7+AP) encoding cDNA. At the same time, by using the plasmid containing the T...
Embodiment 2
[0156] Ligation of full-length fusion gene sequences 1 and 2 with pMD19-T vector
[0157] In order to increase the success rate of expression vector construction, the full-length fusion gene sequences 1 and 2 were first ligated and transformed with the pMD19-T vector, and then the successfully transformed clones were subcloned into the expression vectors pTWIN1 and pET-32a to construct the full-length Prokaryotic expression vector of long fusion gene sequences 1 and 2.
[0158] (1) Experimental materials, reagents and equipment
[0159] 1. Materials: Full-length fusion gene sequences 1 and 2 are derived from the results of Example 1.
[0160] 2. See Table 11 for reagents.
[0161] Table 11. Reagents used for linking full-length fusion gene sequences 1 and 2 to pMD19-T vector
[0162] Reagent name Specification batch number Manufacturer pMD19-T Vector 20T CK2401AA TAKARA Top10 Competent Cells 100μl 111108 Tiangen Bio tryptone 500g 8...
Embodiment 3
[0182] Ligation of full-length fusion gene sequences 1 and 2 with prokaryotic expression vector
[0183] The prokaryotic expression vectors pTWIN1 and pET-32a were selected, and the vectors pTWIN1, pET-32a vectors and positive cloning vectors pMD19 / full-length fusion gene sequence 1 and pMD19 / full-length fusion gene sequence 2 were respectively cut with NdeI and PstI. In pTWIN1, the two cohesive ends of the Intern region (about 1549 bp) were removed, and then the same double-digested full-length fusion gene sequences 1 and 2 were inserted into this region; while pET-32a was obtained by removing the two cohesive ends of the expression region of the Trx fusion protein tag. Sticky ends, and then insert the same double-digested full-length fusion gene sequences 1 and 2 in this region. Therefore, NdeI and PstI double enzyme digestion was used, and the TaKaRa ligation kit was used to ligate and transform into the Top10 competent cells of Tiangen Bio.
[0184] (1) Experimental mater...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com