Cefonicid sodium composition powder for injection
A technology of cefnixime sodium and its composition, which is applied in the field of medicine and medicine manufacturing, can solve the problems of low bioavailability and poor curative effect, and achieve the effect of eliminating active effect and improving antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
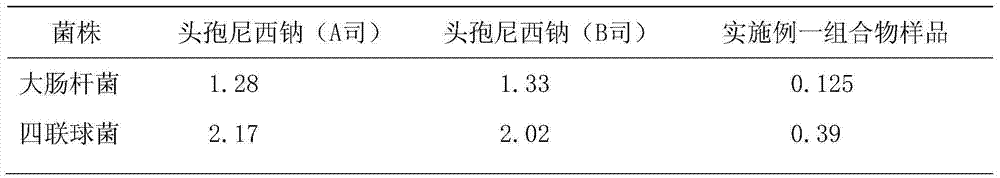
Image
Examples
Embodiment 1
[0033] Embodiment 1, the preparation of the freeze-dried powder injection of the composition of cefnixin sodium for injection, in 1000 pieces.
[0034] Prescription:
[0035] Cefdonix Sodium 80g
[0036] Chitosan Nanoparticles 56g
[0037] Water for injection 2000ml
[0038] 2. Preparation process:
[0039] The chitosan nanoparticle that takes by weighing 56g is slowly added in the water for injection of 2000ml, stirs while adding to dissolve.
[0040] Add 80 g of Cefnixime Sodium and stir to dissolve until clear.
[0041] Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates , according to the calculation of the filling volume of 80 mg per bottle of cefnixime sodium.
[0042] Fill according to the test requirements, put it into a ...
Embodiment 2
[0043] Embodiment 2, the preparation of the freeze-dried powder injection of the cefnixin sodium composition for injection, in 1000 pieces.
[0044] 1. Prescription:
[0045] Cefdonix Sodium 80g
[0046] Chitosan Nanoparticles 68g
[0047] Water for injection 2000ml
[0048] 2. Preparation process:
[0049] The chitosan nanoparticle that takes by weighing 68g is slowly added in the water for injection of 2000ml, stirs while adding to dissolve.
[0050] Add 80 g of Cefnixime Sodium and stir to dissolve until clear.
[0051] Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates , according to the calculation of the filling volume of 80 mg per bottle of cefnixime sodium.
[0052] Fill according to the test requirements, put it into a ...
Embodiment 3
[0053] Embodiment 3, the preparation of the freeze-dried powder injection of the composition of cefnixin sodium for injection, in 1000 pieces.
[0054] Prescription:
[0055] Cefdonix Sodium 80g
[0056] Chitosan Nanoparticles 47g
[0057] Water for injection 2000ml
[0058] 2. Preparation process:
[0059] The chitosan nanoparticle that takes by weighing 47g is slowly added in the water for injection of 2000ml, stirs while adding to dissolve.
[0060] Add 80 g of Cefnixime Sodium and stir to dissolve until clear.
[0061] Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates , according to the calculation of the filling volume of 80 mg per bottle of cefnixime sodium.
[0062]Fill according to the test requirements, put it into a f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com