Catalyst for preparing styrene through ethylbenzene dehydrogenation and preparation method of catalyst
A technology for ethylbenzene dehydrogenation and ethylbenzene dehydrogenation, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. low activity problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 261.5 grams of iron oxide red, 152.0 grams of iron oxide yellow, 70.0 grams of potassium carbonate, 76.8 grams of cerium oxalate, 10.2 grams of ammonium molybdate, 4.0 grams of magnesium oxide, 4.0 grams of graphite, and 10.6 grams of carboxymethyl cellulose were stirred in a kneader For 1 hour, dissolve 6.3 grams of cerium ammonium nitrate in an appropriate amount of deionized water, then add it to the kneader, and stir for another half an hour, take out the extruded strips, extrude them into particles with a diameter of 3 mm and a length of 5 to 10 mm, and put them in the oven , baked at 50°C for 2 hours, baked at 100°C for 10 hours, then placed in a muffle furnace, calcined at 200°C for 8 hours, and then calcined at 600°C for 20 hours to obtain the finished catalyst.
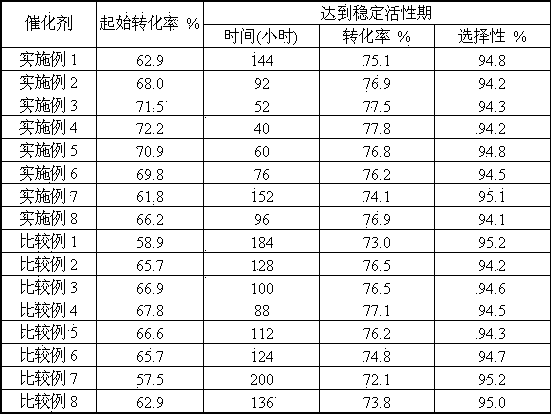
[0025] 100 milliliters of finished catalysts are loaded into the reactor, under normal pressure and liquid space velocity for 1.0 hours -1 , 620°C, and water vapor / ethylbenzene (weight ratio) 2.0 for a...
Embodiment 2
[0028] 253.1 grams of iron oxide red, 100.0 grams of iron oxide yellow, 65.0 grams of potassium carbonate, 75.0 grams of cerium oxalate, 11.5 grams of ammonium tungstate, 11.3 grams of ammonium molybdate, 15.5 grams of magnesium oxide, 11.8 grams of calcium carbonate, 2.0 grams of polystyrene Microspheres and 16.5 grams of carboxymethyl cellulose were stirred in a kneader for 1 hour, 13.0 grams of ammonium cerium nitrate was dissolved in an appropriate amount of deionized water, then added to the kneader, stirred for another half an hour, taken out and extruded into Particles with a diameter of 3 mm and a length of 5 to 10 mm were put into an oven, baked at 50°C for 6 hours, then placed in a muffle furnace, calcined at 300°C for 4 hours, and then calcined at 800°C for 10 hours to obtain a finished catalyst.
[0029] Activity evaluation was carried out according to the evaluation conditions of Example 1, and the test results are listed in Table 2.
[0030]
Embodiment 3
[0032] 213.0 grams of iron oxide red, 90.0 grams of iron oxide yellow, 70.0 grams of potassium carbonate, 73.0 grams of cerium oxalate, 16.3 grams of ammonium molybdate, 5.0 grams of calcium hydroxide, 9.8 grams of magnesium oxide, 5.0 grams of hydroxyethyl cellulose and 20.3 grams Stir carboxymethyl cellulose in a kneader for 1 hour, dissolve 20.0 grams of ammonium cerium nitrate in an appropriate amount of deionized water, then add it to the kneader, and stir for another half an hour, take out the extrusion, and extrude it into a diameter of 3 mm and a length of The particles of 5-10 mm are placed in an oven, baked at 50°C for 2 hours, and baked at 100°C for 10 hours, then placed in a muffle furnace, calcined at 350°C for 4 hours, and then calcined at 820°C for 5 hours to obtain a finished catalyst.
[0033] Activity evaluation was carried out according to the evaluation conditions of Example 1, and the test results are listed in Table 2.
[0034]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com