Synthesis method of valsartan
A synthesis method and technology of valsartan are applied in the field of compound preparation, and can solve the problems of large steric hindrance of tetrazolium group and trityl group, low actual yield of C-C coupling, influence on yield of final product, and the like, Achieve the effect of low cost, easy procurement and reduction of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
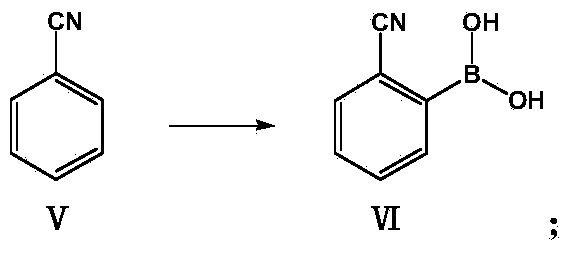
[0034] The valsartan synthetic route of the embodiment of the present invention is as follows:
[0035]
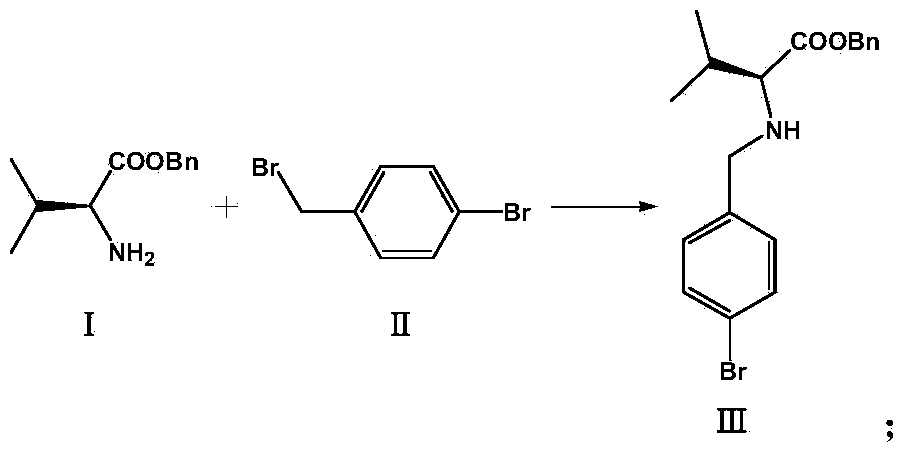
[0036] 1. Synthesis of compound (Ⅲ)
[0037] Mix L-valine benzyl ester (I) (10g, 0.048mol) and potassium carbonate (39.7g, 0.288mol) with 200ml xylene, then add p-bromobenzyl bromide (II) (14.5g, 0.058mol ), heated to reflux, reacted at constant temperature for 2 hours, cooled the system to 5°C, filtered, washed the filter cake twice with cold xylene, and dried to obtain 14.3 g of compound (Ⅲ), with a yield of 98.7%.
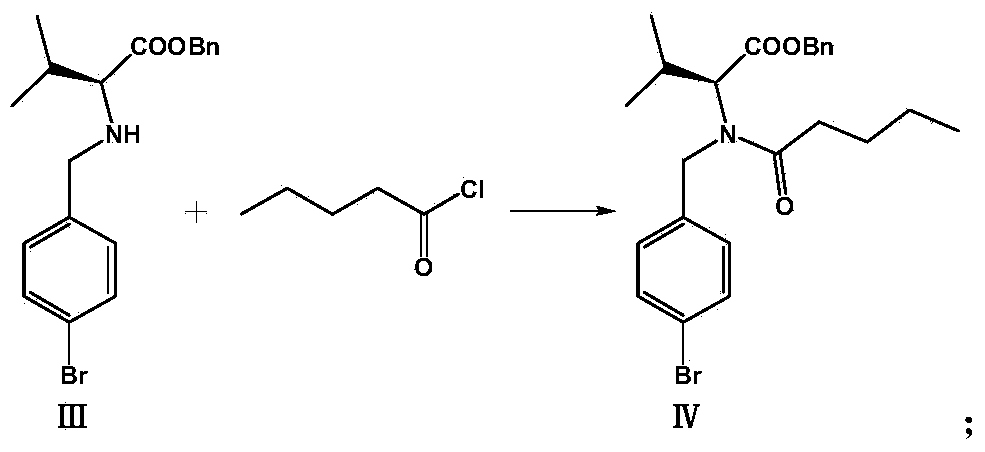
[0038] 2. Synthesis of (S) 2-[(4-bromobenzyl)-pentanamido]-3-methylbutyric acid benzyl ester (Ⅳ)
[0039] Compound (Ⅲ) (14.3 g, 0.048 mol) was dissolved in an appropriate amount of xylene, 11.7 g of 30% NaOH solution, kept at a constant temperature of 40°C, and 7.6 g (0.063 mol) of n-valeryl chloride was slowly added dropwise within 30 minutes under vigorous stirring, and After the addition was complete, the reaction was carried out for another 50 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com