Preparation and storage method of chicken erythrocyte
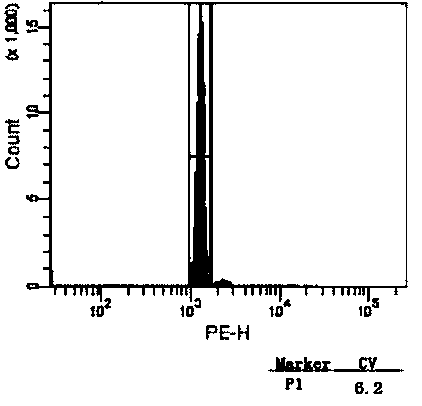
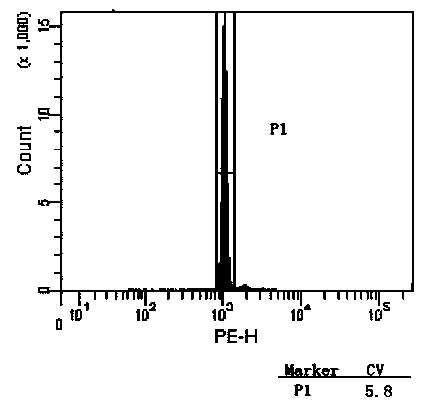
A chicken red blood cell and storage method technology, applied in the field of reagents for medical testing, can solve the problems of inability to remove red blood cells, white blood cells, adhered red blood cells and cell fragments, impurity of chicken red blood cells, non-compliance, etc., so as to reduce the cost of cell preparation and avoid complicated Steps, the effect of reducing the CV value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be described in detail below with reference to the accompanying drawings and in combination with embodiments.
[0031] Unless otherwise specified, the reagents used in the following examples are analytical reagents.
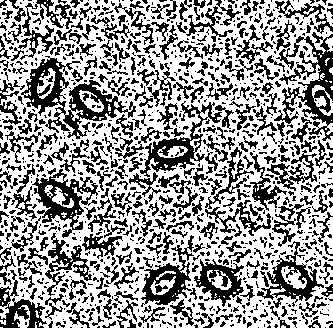
[0032] 1. Chicken red blood cell extraction and purification
[0033] (1) Temporarily store the freshly collected chicken red blood cells anticoagulated with heparin sodium at 4°C, and separate them as soon as possible.
[0034] (2) Mix anticoagulated venous blood with 3% gelatin solution at a ratio of 1:1, and let it stand to separate the solution.
[0035] Preferably, the gelatin solution formula is: 30 g of high-quality gelatin, 0.27 g of potassium dihydrogen phosphate, 1.42 g of disodium hydrogen phosphate, 8 g of sodium chloride and 0.2 g of potassium chloride, dissolved in 1 L of deionized water, heated in a boiling water bath Dissolve and store at 4°C until use.
[0036] (3) Remove the upper layer solution, and resuspen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com