Method and product for establishing conductive hydrogel ultrathin membrane by using layered assembly and electrochemical technology
A technology of conductive hydrogel and electrochemical technology, which is applied in the field of preparation of conductive hydrogel ultra-thin films, can solve problems such as application limitations, and achieve good biocompatibility, excellent biocompatibility, uniform distribution and variable content. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Prepare a sodium chloride buffer solution with a molar concentration of 0.15M;
[0035] Prepare a polylysine (PLL) solution with a mass concentration of 0.5 mg / mL in a buffer solution, and stir until the PLL is fully dissolved;
[0036] Prepare a hyaluronic acid (HA) solution with a mass concentration of 1 mg / mL in a buffer solution, and stir until the HA is fully dissolved;
[0037] (2) Soak the electrode in the PLL solution for 8 minutes, and then wash it with the buffer solution in step (1) for 3 times; then soak it in the HA solution for 8 minutes, and then wash it with the buffer solution in step (1) 3 times (step (2) constitutes the preparation of a double layer);
[0038] (3) Prepare a mixed solution of pyrrole with a mole fraction of 50mM and 10mM sodium p-toluenesulfonate in a buffer solution, and stir until the pyrrole and sodium p-toluenesulfonate are fully dissolved;
[0039] (4) Repeat the process of (2) 16 times to prepare (PLL / HA) coated 16 The ele...
Embodiment 2~5
[0042] The same preparation method as in Example 1 was adopted, except that the polymerization reaction time in step (5) was different, and the polymerization reaction time in Examples 2-5 were 200 s, 300 s, 400 s and 500 s, respectively.
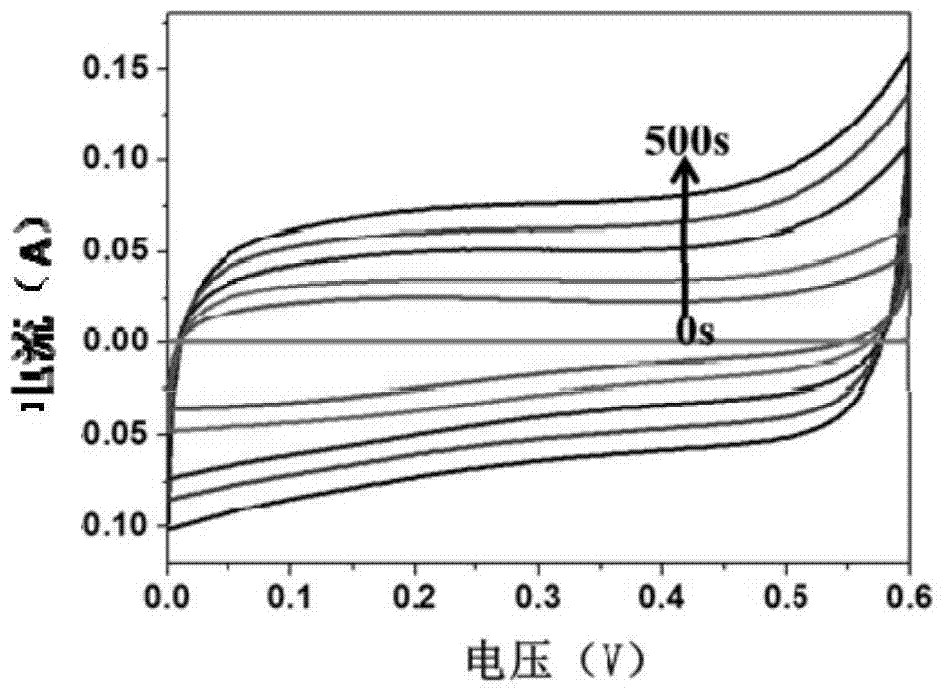
[0043] figure 1 It is the cyclic voltammetry curve (scanning speed is 50mV / s) of the conductive hydrogel ultra-thin films prepared respectively in Examples 1-5, in the figure, observe figure 1It can be found that the cyclic voltammetry current is proportional to the electropolymerization time, and the conductivity of the conductive hydrogel ultrathin film gradually increases with the increase of the electrification time.
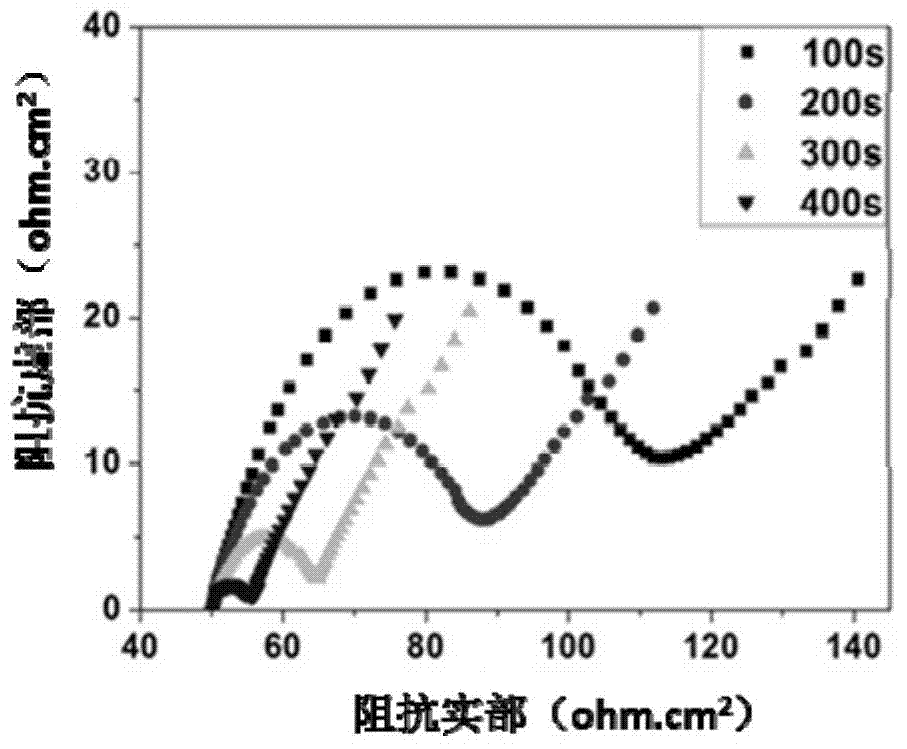
[0044] figure 2 It is the Nyquist impedance curve of the conductive hydrogel ultrathin films prepared respectively in Examples 1-4. Observed figure 2 It can be found that the Nyquist radius in the high-frequency region decreases with the increase of electropolymerization time, indicating that the electron transfer in...
Embodiment 6
[0050] (1)~(4) Perform 5 steps (1)~(4) in the same example;
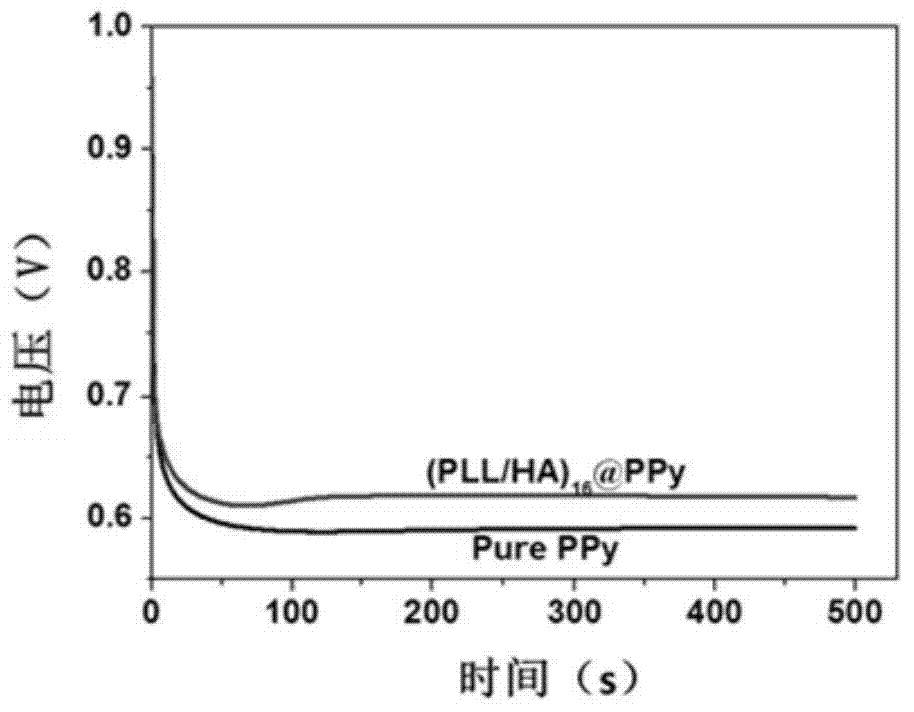
[0051] (5) Put the electrode soaked in step (4) into the electrochemical cell containing the solution in step (3), and connect the saturated potassium chloride electrode, platinum sheet and conductive electrode to the reference electrode of CHI660DE electrochemical workstation. On the interface of the specific electrode, counter electrode, and working electrode; in the chronopotentiometry on the electrochemical workstation, set the current parameter to 0.05mA, limit the maximum initial voltage to 1V, stop the reaction for 120s after energizing for 50s, and stop the reaction for 120s after energizing again for 50s Stop the reaction for 240s, and stop the reaction after energizing again for 50s to obtain a conductive hydrogel ultra-thin film containing a certain amount of polypyrrole, which is stored in a buffer solution.
[0052] According to the Sauerbrey formula,
[0053] In the formula, △F is the change of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com