Method for continuous preparation of sodium azide
A technology of sodium azide and nitrogen oxides, applied in the direction of azide acid/azide/halide azide, etc., can solve the problems of waste water, large inorganic salts, etc., achieve low energy consumption, simple operation, easy to widely popularize Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
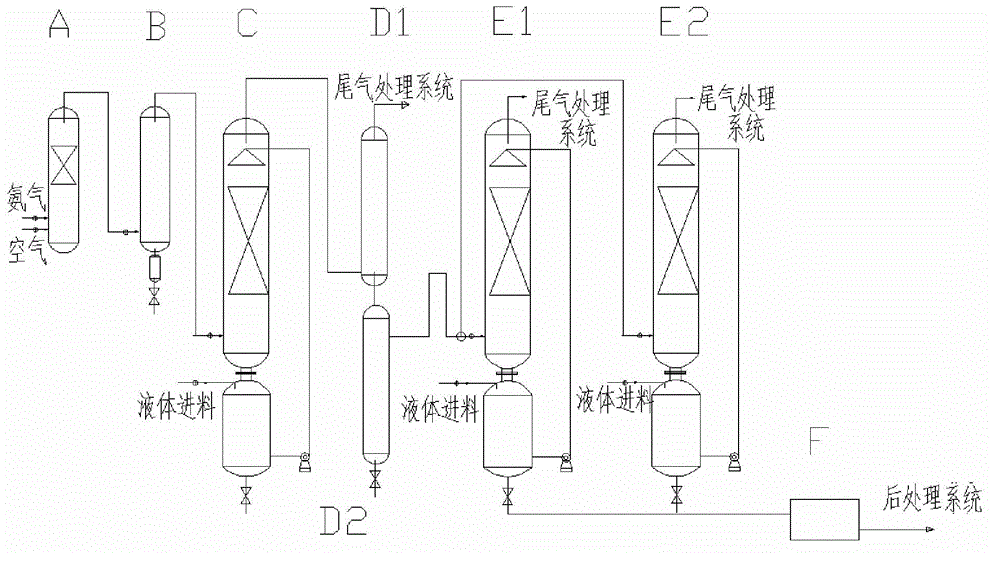
[0038] The present embodiment provides a kind of method for continuously preparing sodium azide, and the method adopts such as figure 1 The continuous reaction device shown realizes the continuous preparation of sodium azide, and described continuous reaction device comprises ammonia oxidation furnace A, and the top of ammonia oxidation furnace A communicates with the feed inlet of heat exchanger B, and the top of heat exchanger B The discharge port is connected to the bottom of the tower body of the tower esterification reactor C, and the tower still of the tower esterification reactor C transfers the tower liquid to the top of the tower body through a pump, and the top of the tower body of the tower esterification reactor C It is connected with the bottom of the condenser D1, the bottom of the condenser D1 is connected with the top of the heater D2, the top of the heater D2 is connected with the feed port of the three-way valve, and one discharge port of the three-way valve i...
Embodiment 2
[0046] The present embodiment provides a kind of method for continuously preparing sodium azide, and the method adopts such as figure 1 Shown continuous reaction device realizes the continuous preparation of sodium azide, and continuous reaction device is identical with embodiment 1.
[0047] The method for continuously preparing sodium azide specifically comprises the following steps:
[0048] Step 1: Pass ammonia and air respectively from the bottom of ammonia oxidation furnace A to carry out catalytic oxidation reaction, and the generated nitrogen oxides are passed to the bottom of heat exchanger B for heat exchange, and the temperature of nitrogen oxides is reduced to 30°C-40°C °C, wherein: the reaction temperature of ammonia oxidation is controlled within the range of 700 °C to 720 °C, the pressure is within the range of 0.1 to 0.15 Mpa, the molar ratio of ammonia to oxygen is 1:1.5, and the catalyst is a platinum-rhodium catalyst;
[0049] Step 2, the nitrogen oxides co...
Embodiment 3
[0054] The present embodiment provides a kind of method for continuously preparing sodium azide, and the method adopts such as figure 1 Shown continuous reaction device realizes the continuous preparation of sodium azide, and continuous reaction device is identical with embodiment 1.
[0055] The method for continuously preparing sodium azide specifically comprises the following steps:
[0056] Step 1: Pass ammonia and air respectively from the bottom of ammonia oxidation furnace A to carry out catalytic oxidation reaction, and the generated nitrogen oxides are passed to the bottom of heat exchanger B for heat exchange, and the temperature of nitrogen oxides is reduced to 30°C-40°C °C, wherein: the reaction temperature of ammonia oxidation is controlled within the range of 700 °C to 720 °C, the pressure is within the range of 0.1 to 0.15 Mpa, the molar ratio of ammonia to oxygen is 1:1.5, and the catalyst is a platinum-rhodium catalyst;
[0057] Step 2, the nitrogen oxides co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com