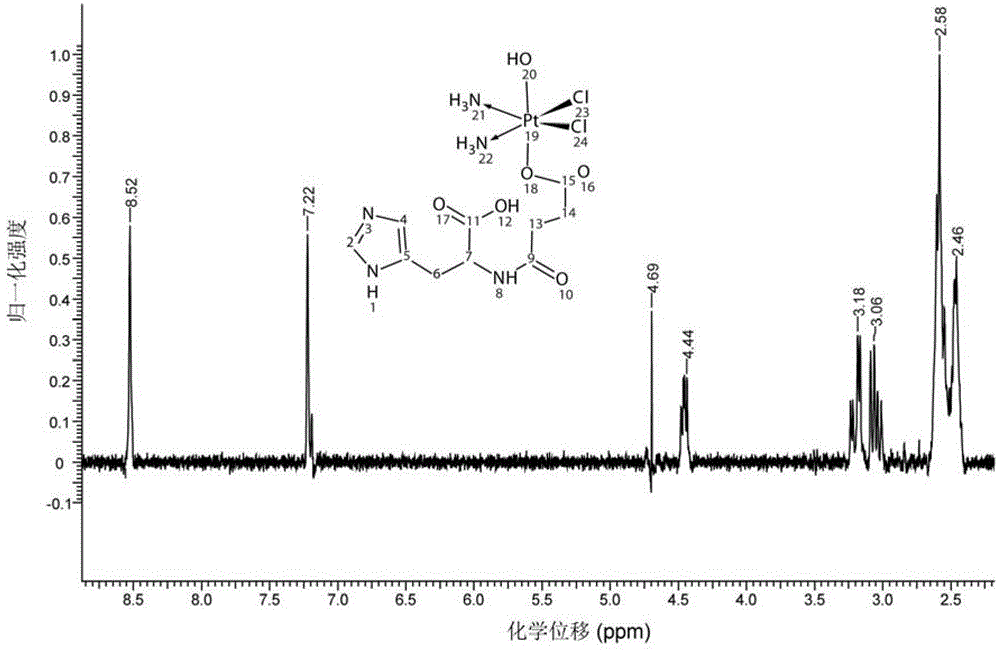
Platinum complexes, protein complexes prepared from same, and preparation method of protein complexes
A technology of complexes and complexes, applied in chemical instruments and methods, pharmaceutical formulations, compounds containing elements of group 8/9/10/18 of the periodic table, etc., to achieve good tumor cell growth inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: construct the expression vector of azurin
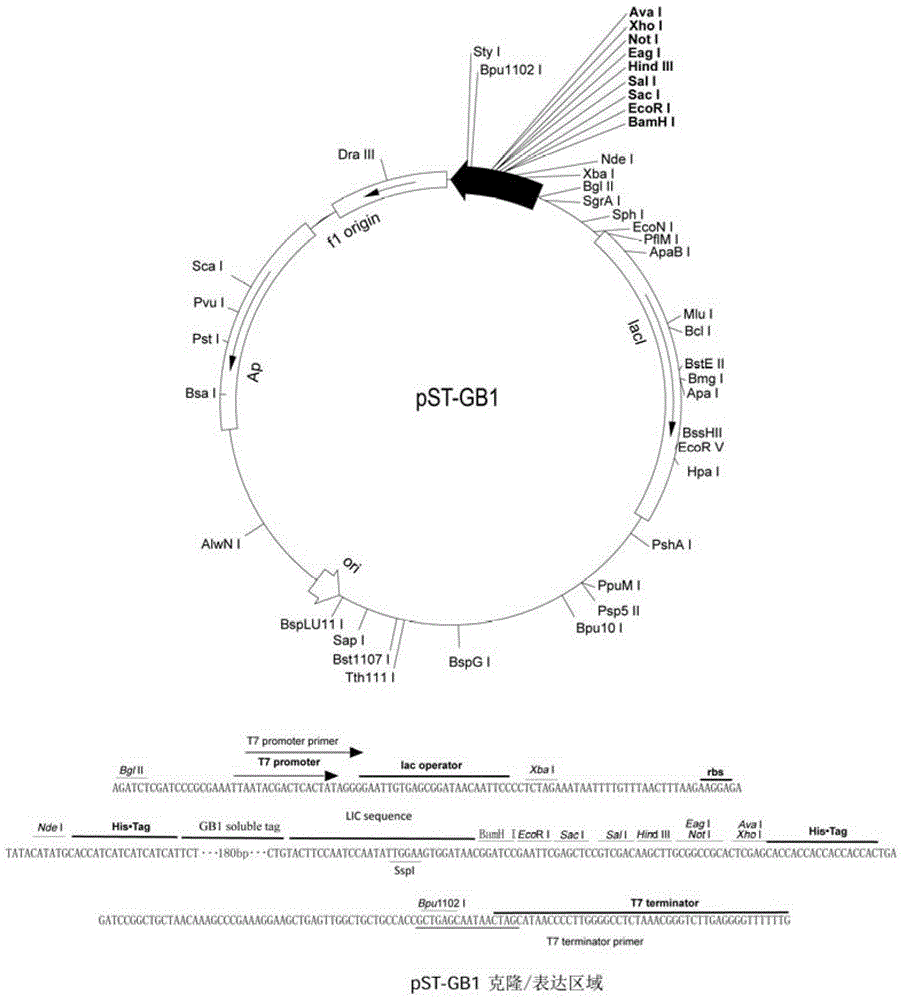
[0038] Use SspI enzyme (purchased from Takara Company) to digest and contain an N-terminal containing (His) 6 -tag, the prokaryotic expression vector pST-GB1 of the GB1 tag containing the TEV restriction site at the C-terminus (see the attached map for the map) figure 1 ), enzyme digestion for 4 hours (see Table 1-1 below for specific conditions). Agarose gel electrophoresis, and gel recovery to obtain the pST-GB1 vector after SspI digestion. Among them, the pST-GB1 vector is a plasmid transformed by our laboratory on the basis of the commercial pET21a. The sequence between the NdeI and BamHI restriction sites on pET21a is excised, and a link containing (His) 6 - a tag, a GB1 solubilizing tag and a sequence of a LIC sequence containing a SspI enzyme cleavage site. With SEQ ID NO: 3 as the forward primer, and SEQ ID NO: 4 as the reverse primer (SEQ ID NO: 3 and SEQ ID NO: 4 were purchased from Shanghai Sangon B...
Embodiment 2
[0050] Example 2: Construction of a molecular cloning vector for an azurin mutant protein by site-directed mutagenesis.
[0051] Using the site-directed mutagenesis technique to the plasmid obtained in Example 1, the histidine on the copper binding site of azurin is mutated into the hydrophobic amino acid glycine to obtain the plasmid of the azurin mutant, and the specific steps are as follows:
[0052] With SEQ ID NO: 5 as the forward primer, and SEQ ID NO: 6 as the reverse primer (SEQ ID NO: 5 and SEQ ID NO: 6 were both purchased from Shanghai Sangon Bioengineering Company), obtained in Example 1 The plasmid was used as a template, using Takara PrimerSTAR TM HS DNA Polymerase with GC Buffer kit for PCR amplification (see Table 2-1 and Table 2-2 for specific conditions); gel recovery of PCR products after agarose gel electrophoresis; process PCR with DpnI enzyme (purchased from Takara) at 37°C The product was produced for 1 hour (see Table 2-3); the digested PCR product was...
Embodiment 3
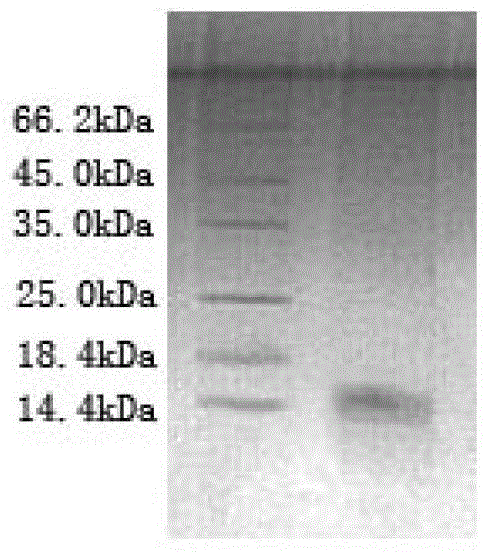
[0060] Example 3: Expression and purification of azurin mutants
[0061] Transfer the plasmid obtained in Example 2 into BL21 (DE3) competent bacteria, spread the plate, pick a single clone transformant, expand and cultivate to 1 liter of LB medium (each liter of medium contains 0.1 mg of ampicillin) ) cultured; to OD 600 When =0.8, add copper chloride (final concentration is 20 μmol / L) and isopropyl-β-D-thiogalactopyranoside (IPTG, final concentration is 0.2mmol / L), and induce at 25°C for 10 hours .
[0062] Harvest bacteria at 25°C at 4,000 rpm for 20 minutes; resuspend bacteria in Ni-NTA resuspension buffer (30 ml buffer per liter of bacteria); sonicate bacteria at 16,000 rpm at 4°C Centrifuge the cell disruption solution for 30 minutes, and filter the supernatant to obtain the supernatant of the cell disruption solution.
[0063] Pour the supernatant into a Ni-NTA affinity chromatography resin column (Shanghai Sangon Bioengineering Co., Ltd.), and shake on a rotary shak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com