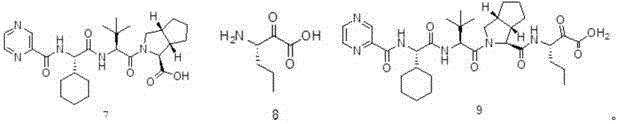
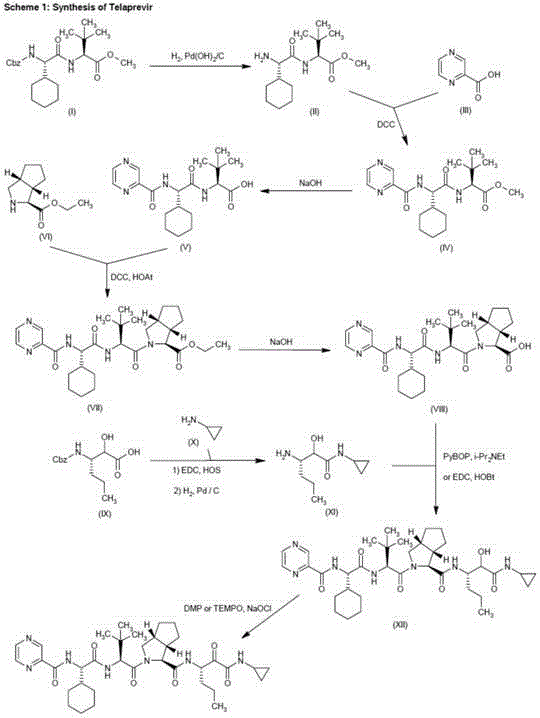
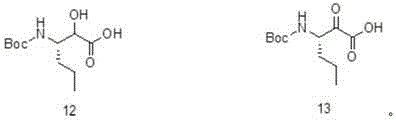Telaprevir preparation method
A technology of telaprevir and compounds, which is applied in the field of preparation of telaprevir, can solve the problem of high cost, achieve the effects of improving synthesis yield, mild reaction conditions, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The embodiment of the present invention discloses a preparation method of telaprevir. Those skilled in the art can refer to the content of this article to appropriately improve the process parameters to achieve. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method of the present invention has been described through preferred embodiments, and relevant personnel can obviously make changes or appropriate changes and combinations to the method described herein without departing from the content, spirit and scope of the present invention to realize and apply the technology of the present invention .
[0030] For realizing the purpose of the present invention, the present invention adopts following technical scheme:
[0031] A preparation method of telaprevir, comprising:
[0032] Step 1: Condensation of 2-carboxypyrazi...
Embodiment 1
[0067] Embodiment 1: the preparation of compound 3
[0068] Dissolve pyrazine 2-carboxylate (2.48g, 20mmol) and HONb (3.942g, 22mmol) in 50mL THF, add DCC (4.539g, 22mmol) under ice-bath conditions, keep stirring in the ice-bath for 1h, remove the ice-bath, Stir at room temperature for 2h, filter, and the filtrate is set aside.
[0069] L-cyclohexylglycine (3.144g, 20mmol) and Na 2 CO 3 (2.12g, 20mmol) was dissolved in 100mL H 2 O, under ice-bath conditions, the above filtrate was added dropwise while stirring, and after the dropwise addition was completed, the reaction was carried out at room temperature for 2 h. Stop the reaction, concentrate THF in vacuo, add 50mL EA, adjust the pH to 3 with saturated citric acid, stir for 0.5h, separate the organic phase, wash with saturated brine three times, and dry over anhydrous sodium sulfate. Concentrated in vacuo, the obtained oil was recrystallized with ether to obtain 4.27g of white powdery solid, yield: 81%, MS: 264(M+1), 286...
Embodiment 2
[0070] Embodiment 2: the preparation of compound 5
[0071] Compound 3 (2.63g, 10mmol) and HONb (1.97g, 11mmol) were dissolved in 30mL THF, DCC (2.269g, 11mmol) was added under ice-bath conditions, kept stirring in the ice bath for 1h, removed the ice bath, and stirred at room temperature 2h, filtered, and the filtrate was set aside.
[0072] L--tertiary leucine (1.311g, 10mmol) and NaHCO 3 (1.768g, 20mmol) was dissolved in 50mL H 2 O, under ice-bath conditions, the above filtrate was added dropwise while stirring, and after the dropwise addition was completed, the reaction was carried out at room temperature for 3 h. Stop the reaction, concentrate THF in vacuum, extract twice with petroleum ether, add 25mL EA to the water phase, adjust the pH to 3 with saturated citric acid, stir for 0.5h, separate the organic phase, wash with saturated brine three times, and dry over anhydrous sodium sulfate. Concentrated in vacuo, the resulting foamy solid was recrystallized with PE-EA t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com