Content determination method for 4,5-dimethoxy-1-(methyl amino methyl) benzocyclobutane optical isomer
A technology of methylaminomethyl and benzocyclobutane is applied in the detection field of pharmaceutical intermediate content, which can solve problems such as difficult optical isomer content, achieve great social significance, strong practicability, and solve quality control problems Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Chromatographic column: OD-RH (Chiralcel, 150mm×4.6mm, 5μm) chiral chromatographic column, column temperature 35°C
[0021] Mobile phase: 0.05mol / L potassium hexafluorophosphate solution (adjust the pH value to 2.0 with phosphoric acid solution)-acetonitrile (80:20)
[0022] Detection wavelength: 230nm
[0023] Flow rate: 0.5ml / min
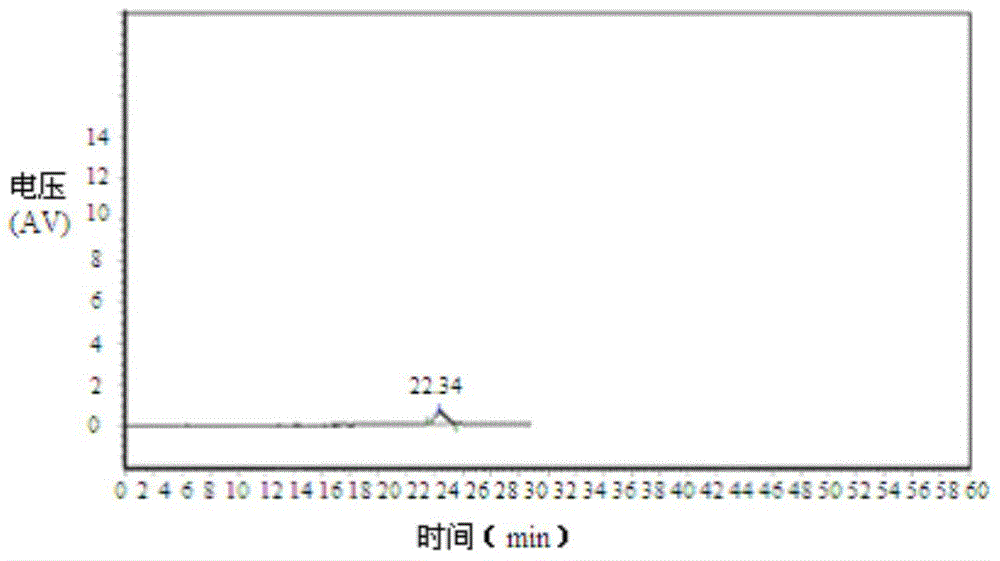
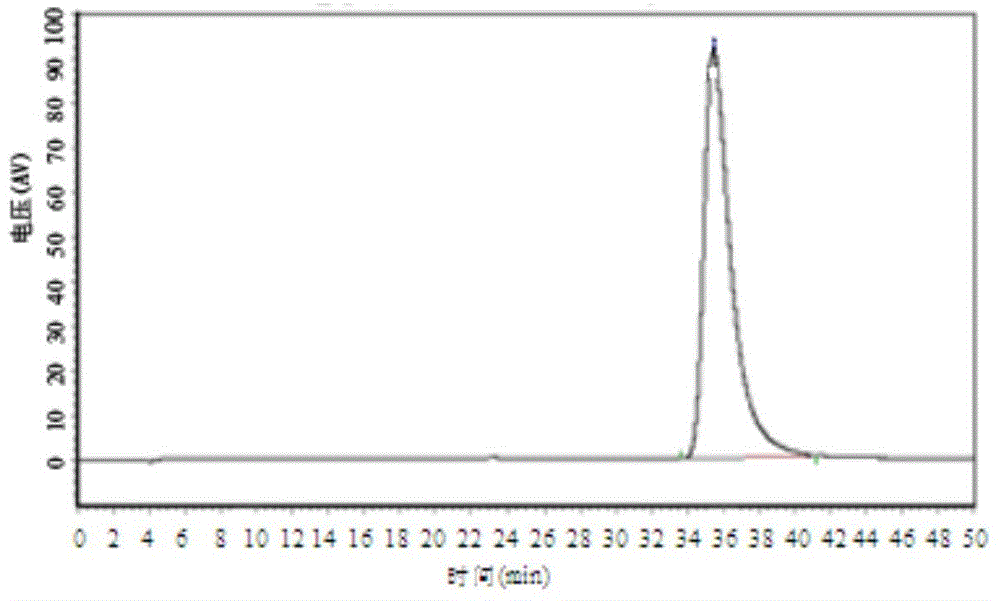
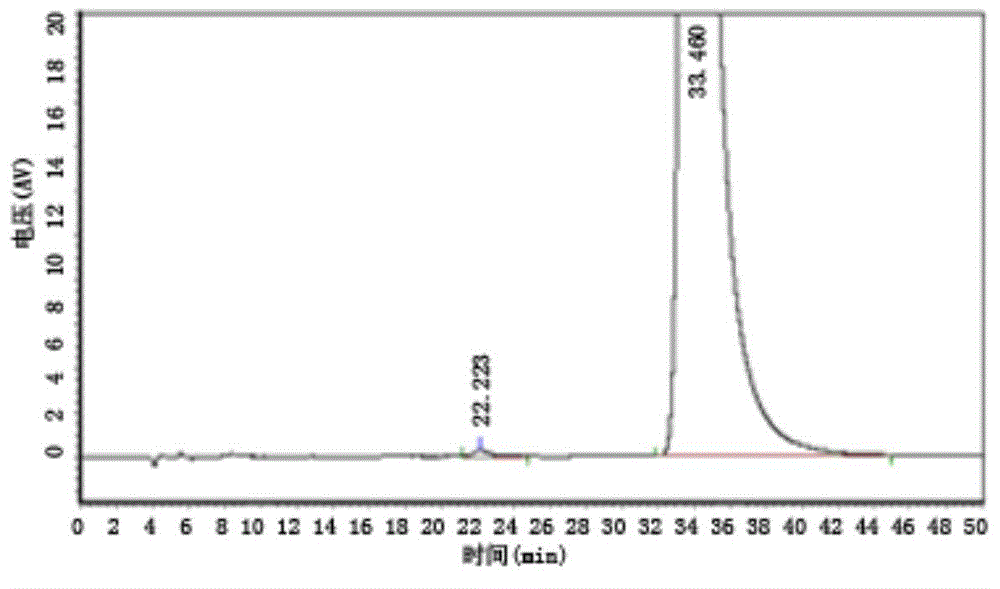
[0024] Take an appropriate amount of 4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane, dissolve and dilute with mobile phase to make a solution containing about 0.1mg per 1ml, as the test solution; Each of 4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane S-isomer and 4,5-dimethoxy-1-(methylaminomethyl)benzene An appropriate amount of cyclobutane R-isomer is dissolved and diluted with mobile phase to make a solution containing about 1 μg per 1 ml, which is used as a reference substance stock solution, and the liquid phase positioning map is as follows: figure 1 and figure 2 As shown; take 4,5-dimethoxy-1-(methylaminomethyl)benzocy...
Embodiment 2
[0026] Chromatographic column: OD-RH (Chiralcel, 150mm×4.6mm, 5μm) chiral chromatographic column, column temperature 25°C
[0027] Mobile phase: 0.05mol / L potassium hexafluorophosphate solution (adjust the pH value to 2.5 with phosphoric acid solution)-methanol (95:5)
[0028] Detection wavelength: 230nm
[0029] Flow rate: 0.2ml / min
[0030] Take an appropriate amount of 4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane, dissolve and dilute with mobile phase to make a solution containing about 0.1mg per 1ml, as the test solution; Take an appropriate amount of 4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane R-isomer, dissolve and dilute with mobile phase to make a solution containing about 1 μg per 1ml, as a reference substance Stock solution; take an appropriate amount of 4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane and R-isomer, dissolve and dilute with mobile phase to make 4,5 - A mixed solution of 0.1 mg of dimethoxy-1-(methylaminomethyl)benzocyclobutane an...
Embodiment 3
[0032] Chromatographic column: OD-RH (Chiralcel, 150mm×4.6mm, 5μm) chiral chromatographic column, column temperature 30°C
[0033] Mobile phase: 0.05mol / L sodium hexafluorophosphate solution (adjust the pH value to 1.8 with phosphoric acid solution)-methanol and ethanol mixture (75:25)
[0034] Detection wavelength: 230nm
[0035] Flow rate: 0.5ml / min
[0036] Take an appropriate amount of 4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane, dissolve and dilute with mobile phase to make a solution containing about 0.1mg per 1ml, as the test solution; Take an appropriate amount of 4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane R-isomer, dissolve and dilute with mobile phase to make a solution containing about 1 μg per 1ml, as a reference substance Stock solution; take an appropriate amount of 4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane and R-isomer, dissolve and dilute with mobile phase to make 4,5 - A mixed solution of 0.1 mg of dimethoxy-1-(methylaminomethyl)b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com