Formula and preparation method of preparation delivered through mucous membrane
A mucosal and formulation technology, applied in the field of biomedicine, can solve the problems of economic unfeasibility of active ingredients of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 protein hydrolyzate
[0032] A: Dissolve 10 grams of lactoferrin in 90 grams of purified water, then adjust the pH of the solution to 2 with 1M hydrochloric acid, heat the resulting solution to 120 ° C, acid hydrolyze, hydrolyze for 1 hour, then cool, and then use 1M hydrochloric acid Adjust the pH to 8.5 with sodium hydroxide, and filter to obtain about 100 grams of a solution of 10% lactoferrin hydrolyzate (10 grams of hydrolyzate in net weight).
[0033] B: 10 g of lactoferrin was dissolved in 90 g of purified water, the resulting solution was adjusted to pH 2 with 1M hydrochloric acid, and then heated to 100° C. for 6 hours for hydrolysis. The hydrolyzate was cooled, and then adjusted to pH 8.5 with 1M sodium hydroxide, and filtered to obtain about 100 g of 10% lactoferrin hydrolyzate (10 g of hydrolyzate in net weight).
[0034] C: Dissolve 10 grams of lactoferrin in 90 grams of purified water, adjust the pH of the resulting aqueous...
Embodiment 2
[0037] Embodiment 2: Exenatide formulation preparation
[0038] 0.5 kg of this formula is prepared according to the following table 1:
[0039] Table 1:
[0040] Element
g / batch
%
MCT (Zhejiang Jiande Qiandao Fine Chemical Industry Co., Ltd.) (Oil)
65
13.00
corn oil (oil)
39.9
7.97
53.2
10.64
Potassium sorbate (water phase)
0.7
0.14
Sodium benzoate (water phase)
0.7
0.14
Methylparaben (oil)
0.7
0.14
Propylparaben (oil)
0.7
0.14
48
9.60
Tween 80 (water phase)
66.6
13.32
3.3
0.66
Exenatide (Shenzhen Haodi Huatuo Biochemical Technology Co., Ltd.)
0.025
0.01
Lactoferrin hydrolyzate solution (see Example 1 D)
221.2
44.24
total
500.0
100.0
[...
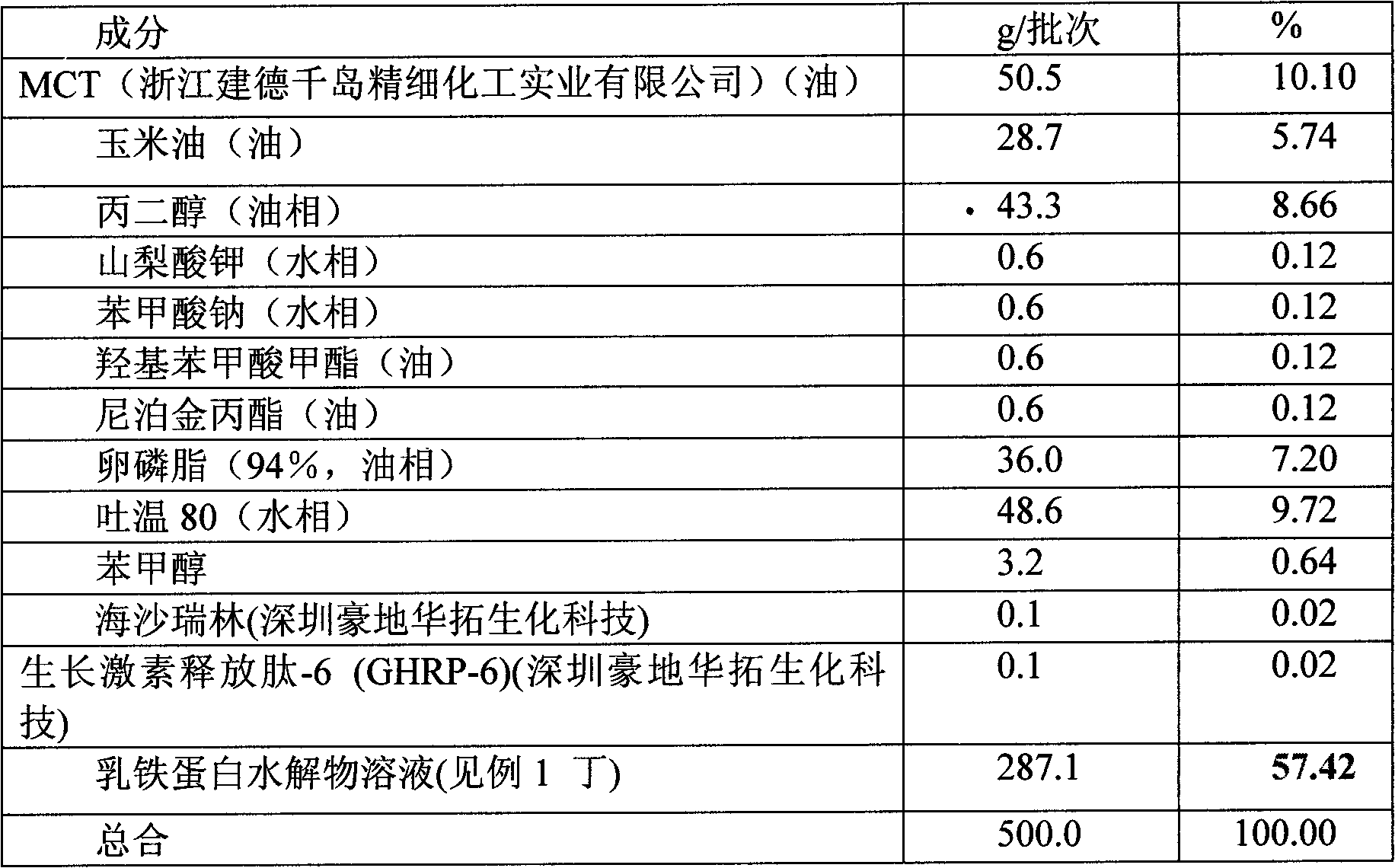
Embodiment 3
[0045] Embodiment 3 mixing component prescription preparation
[0046] 0.5 kg of this formula is prepared according to the following table 2:
[0047] Table 2:
[0048]
[0049] Oil Phase: Add ingredients in the following order: Corn Oil, MCTs, Propylene Glycol, Methyl & Propyl Parabens, and Benzyl Alcohol. The mixture was heated to 55-60°C and all ingredients were dissolved with stirring at 250 RPM. Then add lecithin at 55-60°C, mix and dissolve under stirring at 250RPM.
[0050] Water phase: Lactoferrin hydrolyzed solution, potassium sorbate, sodium benzoate and polysorbate 80 (Tween 80) mixed. The pH was maintained at 8.3. Then heat slowly and stir with a stir bar until all the Tween 80 is dissolved. The temperature was maintained at 25-30°C, and the pH was adjusted to 8.3 to 8.5 with 1M sodium hydroxide. Finally add hexarelin and GHRP-6.
[0051] Emulsification: below 40°C, add the oil phase to the water phase, mix and emulsify. Cool the emulsion and add more wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com