Preparation and value setting method for calibration sample used for X-ray fluorescence analysis calibration
A technique for fluorescence analysis and calibration of samples, which is used in material analysis, analysis of materials, and measurement devices using wave/particle radiation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
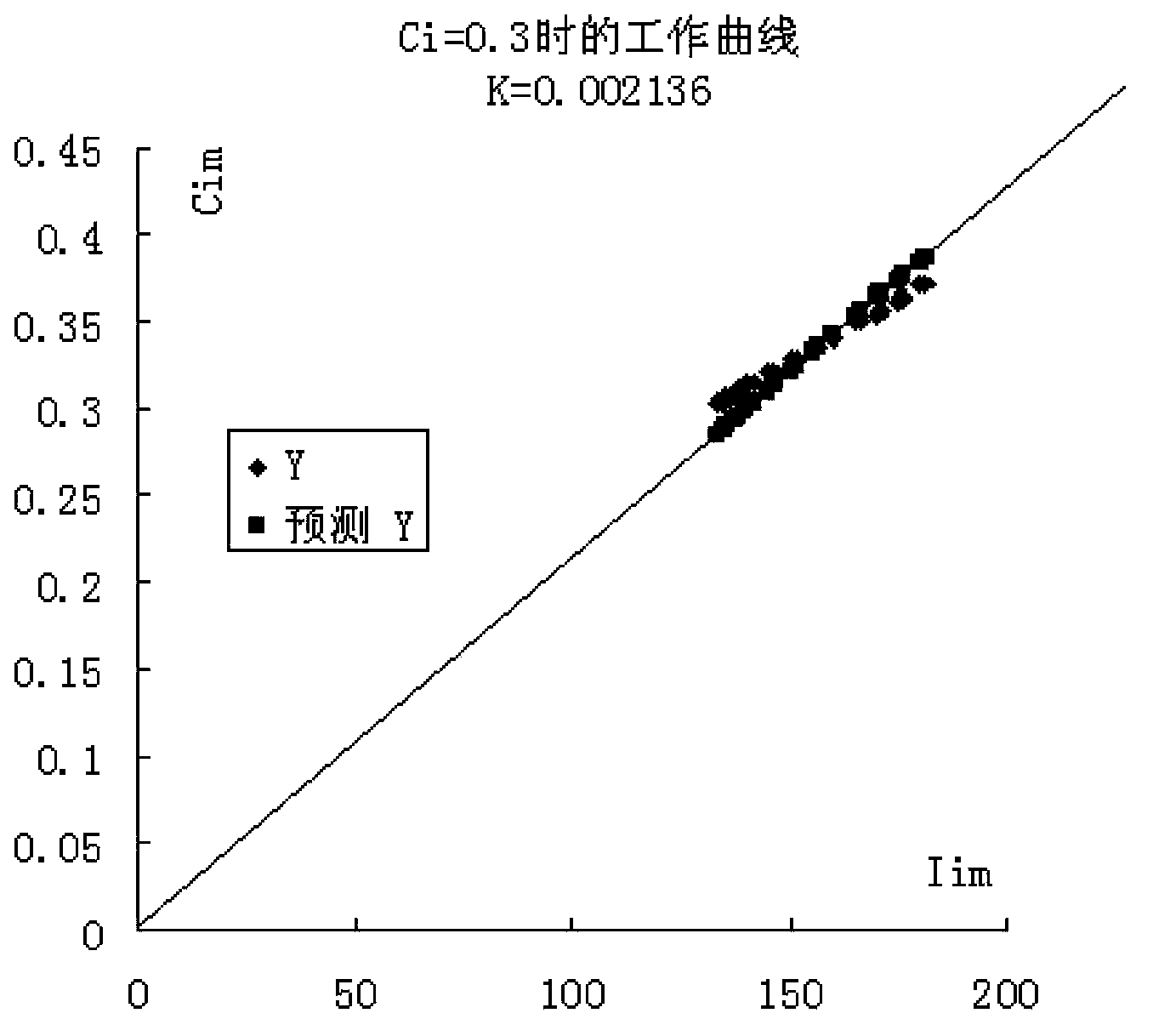
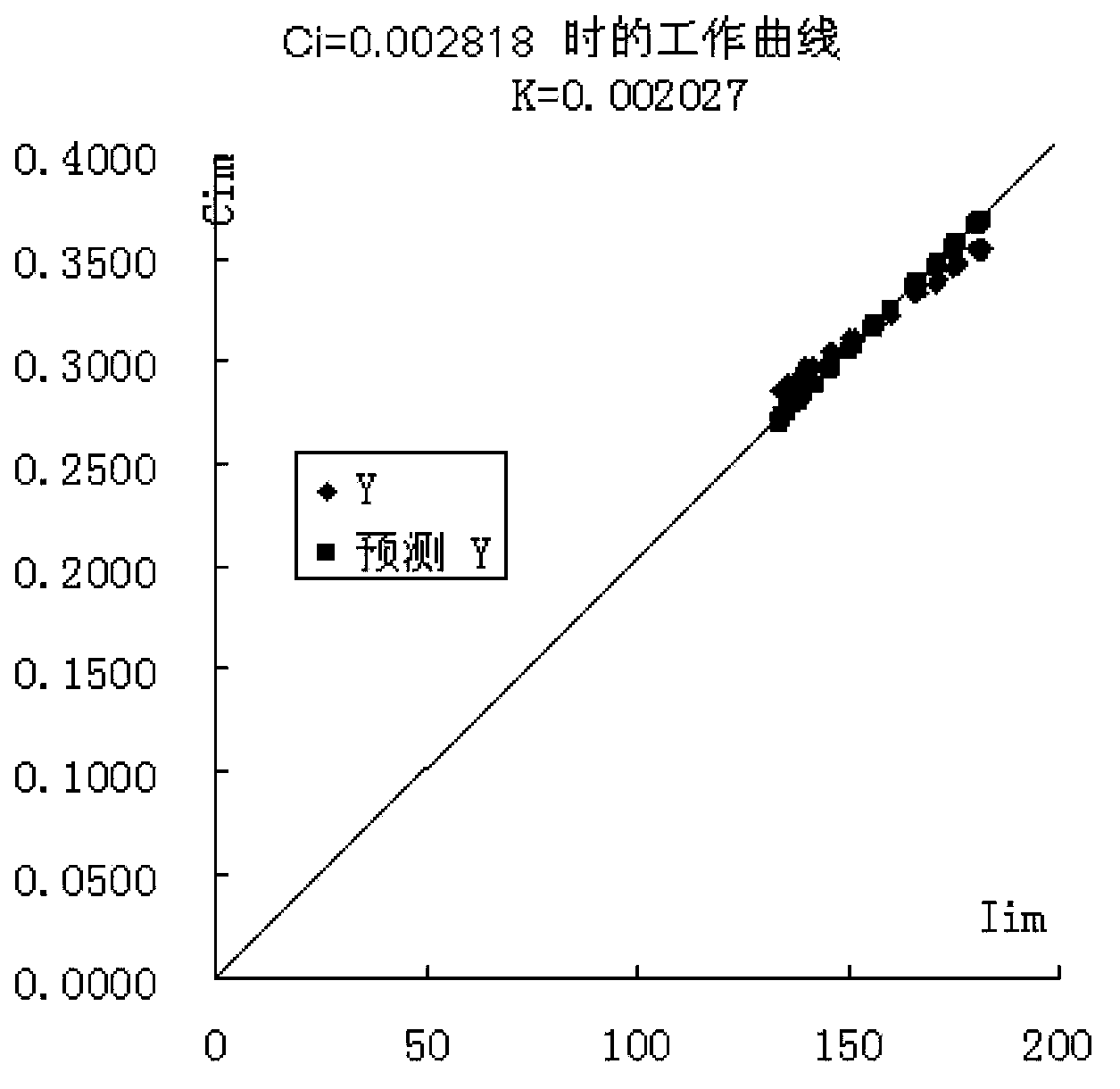
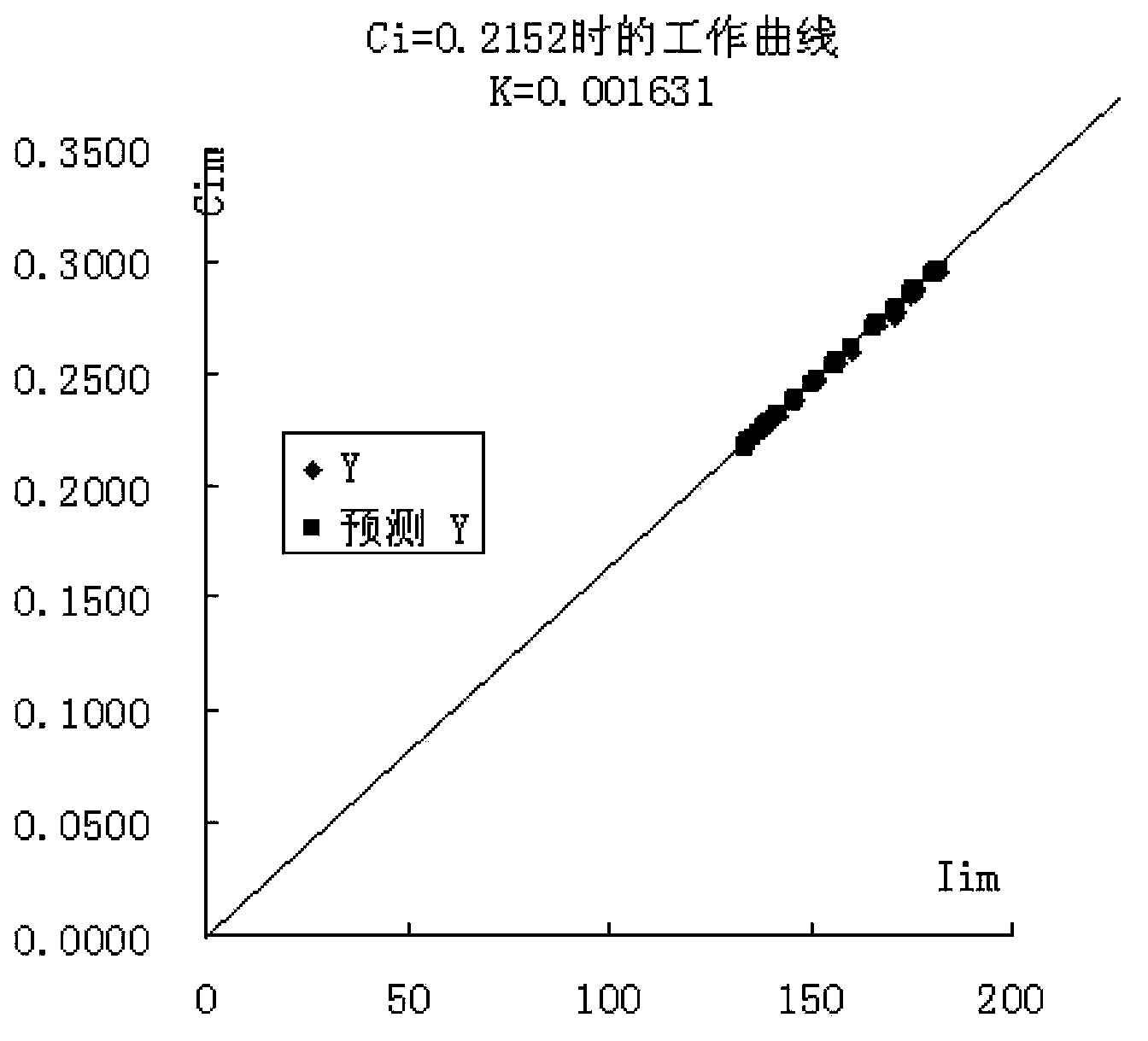
[0030] The invention provides a method for preparing a calibration sample by using an actual sample received by a laboratory as a matrix by adding a reference reagent.
[0031] The method is implemented like this:
[0032] Select a sample S for daily analysis, and prepare S into a uniform powder sample;
[0033] According to the chemical composition of the sample S, prepare corresponding pure reference chemical substances (reference reagents) or related chemical reagents to replace the pure reagents (replacement reagents). For example, for cement samples, the chemical composition is silicon dioxide, aluminum oxide, 11 kinds of compositions such as ferric oxide, calcium oxide, magnesium oxide, sulfur trioxide, potassium oxide, sodium oxide, titanium dioxide, manganese oxide and phosphorus pentoxide, then should prepare 11 kinds of reference reagents or substitute reagents such as silicon dioxide, Since chemical components such as calcium oxide and potassium oxide do not have h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com