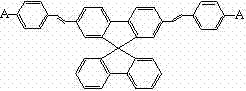
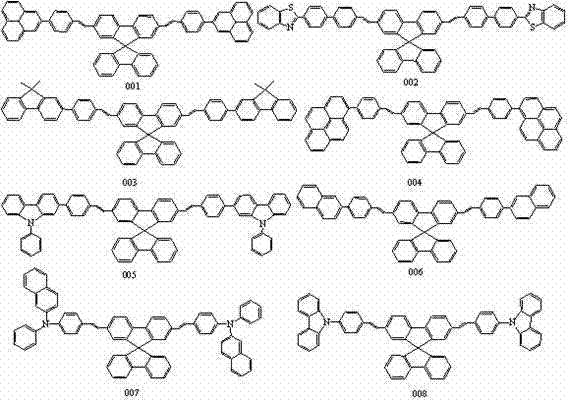
Spirobifluorene olefine organic electroluminescent material and preparation method thereof
A spirobifluorene olefin and luminescent material technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low luminous efficiency and short lifespan, and achieve high luminous efficiency, improved yield and good application value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
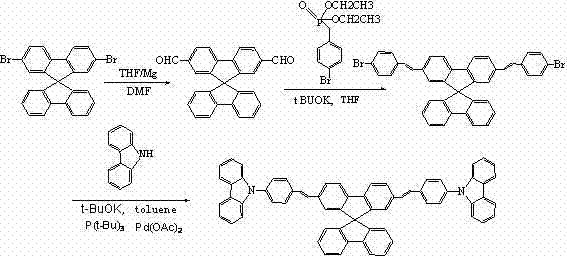
[0021] Embodiment 1: The specific synthetic route of compound 001 is as follows:
[0022]
[0023] (1) Under the condition of nitrogen protection, add 10ml of anhydrous tetrahydrofuran solution, 0.5g of magnesium bar, and 1 grain of iodine into the three-necked flask. After the Grignard reagent triggers, add 4.74g of 2,7-dibromospirobifluorene 10ml of tetrahydrofuran solution in water was reacted for 2 hours in an ice-water bath, 2ml of anhydrous N,N-dimethylformamide was added dropwise to the reaction solution, and then slowly raised to room temperature, and the reaction was continued for 3 hours. After adding 1M hydrochloric acid and 30ml ethyl acetate for liquid separation and extraction, the organic layer was washed with distilled water and saturated brine, dried over sodium sulfate, concentrated, and the resulting crude product was subjected to column chromatography (cyclohexane / dichloromethane=2 / 1), the resulting liquid was rotary evaporated and dried to obtain 2.98 ...
Embodiment 2
[0026] Embodiment 2: The specific synthetic route of compound 002 is as follows:
[0027]
[0028] (1) Under the condition of nitrogen protection, add 10ml of anhydrous tetrahydrofuran solution, 0.5g of magnesium bar, and 1 grain of iodine into the three-necked flask. After the Grignard reagent triggers, add 4.74g of 2,7-dibromospirobifluorene Water tetrahydrofuran solution (10ml) was reacted for 2.1 hours in an ice-water bath, 2ml of anhydrous N,N-dimethylformamide was added dropwise to the reaction solution, and then slowly raised to room temperature, and the reaction was continued for 3.2 hours. After adding 1M hydrochloric acid and 30ml ethyl acetate for liquid separation and extraction, the organic layer was washed with distilled water and saturated brine, dried over sodium sulfate, concentrated, and the resulting crude product was subjected to column chromatography (cyclohexane / dichloromethane=2 / 1), the resulting liquid was rotary evaporated and dried to obtain 2.98 ...
Embodiment 3
[0031] Embodiment 3: The specific synthetic route of compound 003 is as follows:
[0032]
[0033] (1) Under the condition of nitrogen protection, add 10ml of anhydrous tetrahydrofuran solution, 0.5g of magnesium bar, and 1 grain of iodine into the three-necked flask. After the Grignard reagent triggers, add 4.74g of 2,7-dibromospirobifluorene 10ml of water tetrahydrofuran solution was reacted for 2.2 hours under the condition of ice-water bath, and 2ml of anhydrous N,N-dimethylformamide was added dropwise to the reaction solution, then slowly raised to room temperature, and the reaction was continued for 3.4 hours. After adding 1M hydrochloric acid and 30ml ethyl acetate for liquid separation and extraction, the organic layer was washed with distilled water and saturated brine, dried over sodium sulfate, concentrated, and the resulting crude product was subjected to column chromatography (cyclohexane / dichloromethane=2 / 1), the resulting liquid was rotary evaporated and dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com