Red sea bream iridovirus molecular standard sample and its preparation method
A red sea bream iridescent virus and standard sample technology, applied in biochemical equipment and methods, methods based on microorganisms, microorganisms, etc., can solve the problems of aquatic animal disease, difficult to obtain viruses, and undetectable viruses, and achieve good uniformity, Guaranteed quality control, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
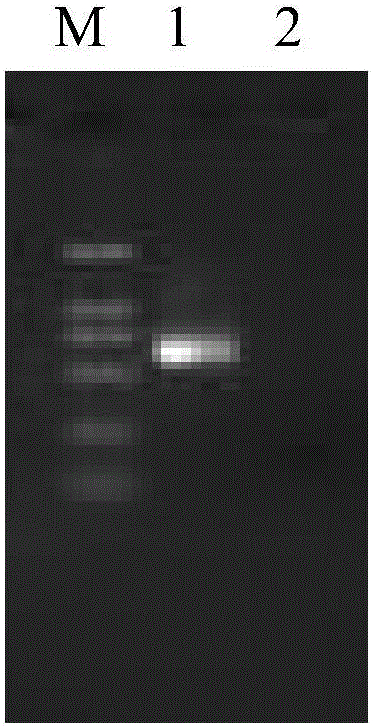
[0027] The preparation of embodiment 1 red sea bream iridovirus molecular standard sample
[0028] 1. Identification of red sea bream iridescent virus
[0029] According to the 2009 version of the OIE Aquatic Animal Diseases Diagnostic Manual Chapter2.3.7 in the fish red sea bream iridovirus polymerase chain reaction method.
[0030] (1) Treatment of tissue disease materials
[0031] Take 50 mg of tissue samples (including brain, liver, spleen and kidney) of diseased fish infected by red sea bream iridescent virus, add 150 μL of CTAB solution, cut into pieces with small scissors, grind and homogenize into a paste, and obtain the tissue homogenate of diseased fish;
[0032] (2) Extraction of DNA: In the diseased fish tissue homogenate obtained in step (1), add CTAB solution (by 2% CTAB, 1.4mol / L NaCl, 20mmol / L EDTA, 20mol / L Tris-HCl pH7.5 To prepare, add mercaptoethanol to a final concentration of 0.25% before use.) to 800 μL, place at 25°C for 2 hours, add 350 μL of extract ...
Embodiment 2
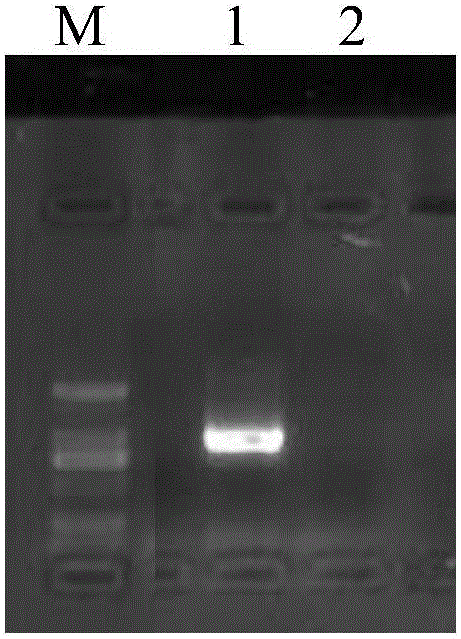
[0067] The homogeneity experiment of embodiment 2 red sea bream iridescent virus molecular standard sample
[0068] The homogeneity determination of red sea bream iridescent virus molecular standard sample is that the red sea bream iridescent virus molecular standard sample prepared by the method of embodiment 1 is randomly divided into two sample groups, i.e. sample group A and sample group B, each sample group has 15 standard samples, each sample was subjected to PCR amplification according to the following reaction conditions, and the concentration of PCR products (ng / μL) was measured. By comparing the concentration changes of PCR products, the uniformity of standard samples was evaluated according to statistical methods. The results are shown in Table 2 , 3 and 4.
[0069] PCR reaction system: Add 1 μL of red sea bream iridescent virus molecular standard sample (containing 1×10 -2 μg DNA), 10 μL of 10-fold buffer for Taq enzyme, 2.5 μL each of primers RSIV-F1 and RSIV-R1,...
Embodiment 3
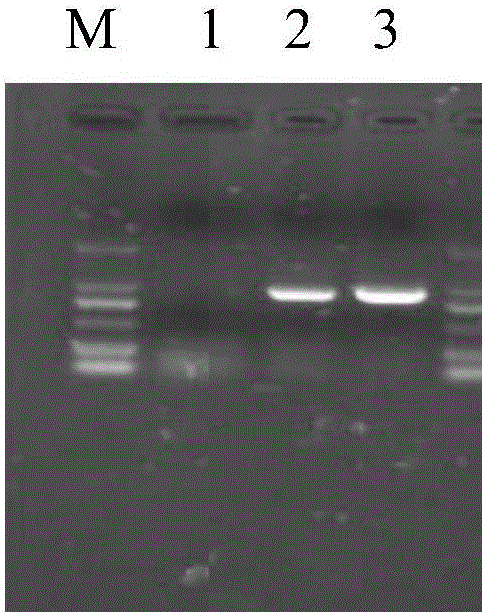
[0078] Stability experiment of embodiment 3 red sea bream iridescent virus molecular standard sample
[0079] The red sea bream iridescent virus molecular standard sample prepared by the method of embodiment 1 is randomly grouped, and each sample is carried out PCR amplification according to the PCR reaction system described in embodiment 2 and the PCR reaction conditions, and measures the PCR product concentration (ng / μL) , by comparing the concentration changes of PCR products, evaluate the stability of standard samples according to statistical methods.
[0080] Two types of stability testing are employed:
[0081] One is the stability test at -20°C. 24 molecular standard samples of red sea bream iridescent virus were randomly selected, and 2 samples were tested every month for 12 consecutive months. The results are shown in Table 5.
[0082] The other is a stability test at a higher temperature of 20°C. 12 molecular standard samples of red sea bream iridescent virus were r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com