Tetraphenyl ethylene derivative and white organic electroluminescent apparatus containing same
A technology of tetraphenylethylene and derivatives is applied in the field of white light organic electroluminescence devices, which can solve the problems of low device service life, poor thermal stability, limited application and the like, and achieve the effects of short synthesis route, easy industrialization and simple preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Synthesis of 4,4',4",4"'-tetra(bromophenyl)ethylene (abbreviated as TBrTPE)
[0042]Under anhydrous and oxygen-free conditions, in 40 mL of anhydrous THF, add 2.6 g of sodium metal, 6.6 mL of titanium tetrachloride and 6.5 g of 4,4’-dibromobenzophenone. Reflux reaction 20h. The reaction solution was acidolyzed with 5% HCl solution, neutralized with 5% NaOH solution, extracted with chloroform, and concentrated under reduced pressure. Pack the column with silica gel and rinse with a mixture of chloroform:petroleum ether (1:3, V:V). The eluent was collected and concentrated to obtain the target product. Yield 48%, MS (m / z): 643.80 (M+). Its molecular formula is:
[0043]
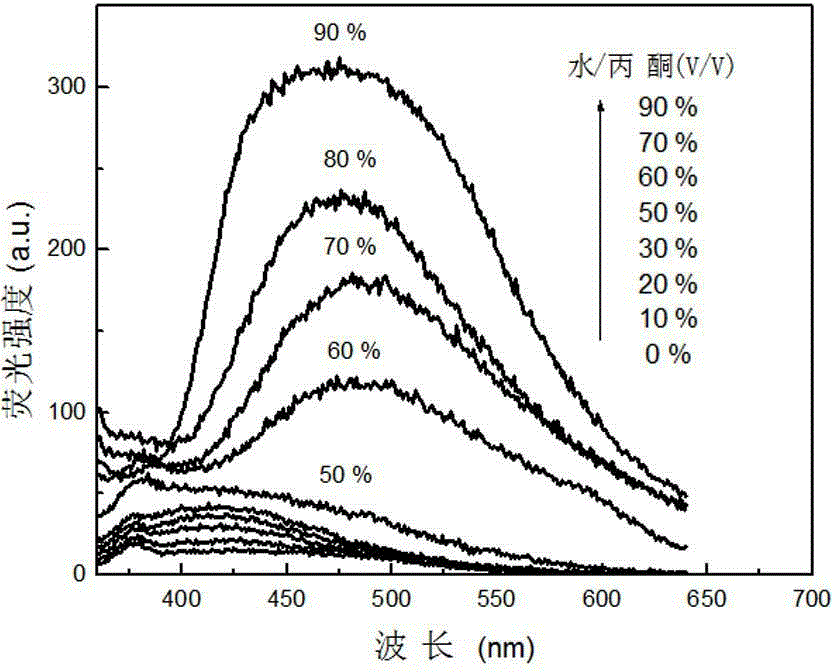
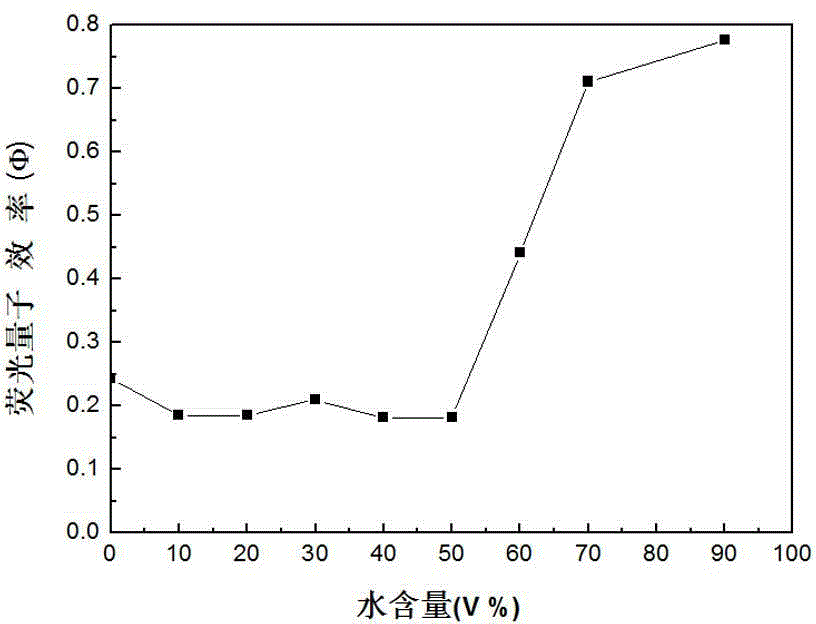
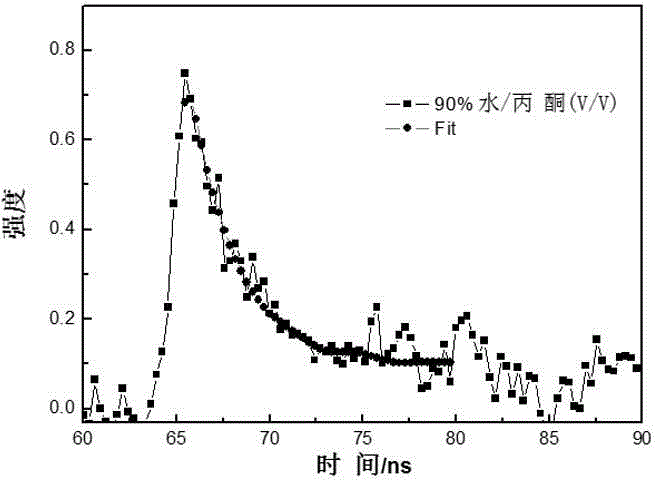
[0044] attached figure 1 It is the change diagram of the absorption spectrum of the above TBrTPE in different proportions of acetone / water mixed solvents, where the volume fraction of water is 0% to 90%; it can be seen that with the increase of water content, the absorption peak position...
Embodiment 2
[0045] Example 2 Synthesis of 4,4',4",4"'-tetra(chlorophenyl)ethylene (TClTPE for short)
[0046] The synthesis method is the same as that in Example 1, except that 4,4'-dibromobenzophenone is changed to 4,4'-dichlorobenzophenone. Yield 48%, MS (m / z): 468.00 (M + ). Yield 42%. The melting point is 267°C. Its molecular formula is:
[0047]
[0048] attached Figure 5 It is the fluorescence spectrogram of above-mentioned TClTPE, wherein (a) is solid state, (b) is that TClTPE is doped in PMMA, (c) is the thin film that TClTPE is spin-coated on glass substrate and makes; As can be seen, this compound has Multiple light strips.
Embodiment 3
[0049] Example Three 4,4',4",4"'-Tetrakis(nitrophenyl)ethylene (referred to as TNO 2 TPE) synthesis
[0050] The synthesis method is the same as that in Example 1, except that 4,4'-dibromobenzophenone is changed to 4,4'-dinitrobenzophenone. Yield 48%, MS (m / z): 512.10 (M + ). Granular white crystals, 42% yield. The melting point is 267°C. Its molecular formula is:
[0051]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com