Gold (III) complex-perylene diimide derivative, and fluorescent sensing tube and tubular fluorescent sensor prepared from same
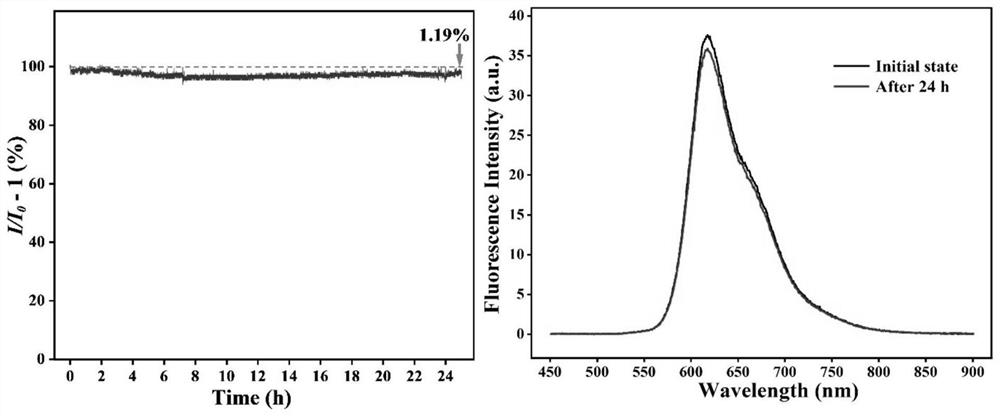
A perylene diimide and fluorescent sensor technology, which is applied in the field of small molecule fluorescent sensing materials, can solve the problem of unreasonable distribution of the gas flow field of the substance to be detected, the large volume of the gas chamber of the laminated fluorescent sensor, and the reduction of the luminous efficiency of the material and other issues, to achieve excellent photothermal stability, sensing selectivity and sensitivity improvement, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
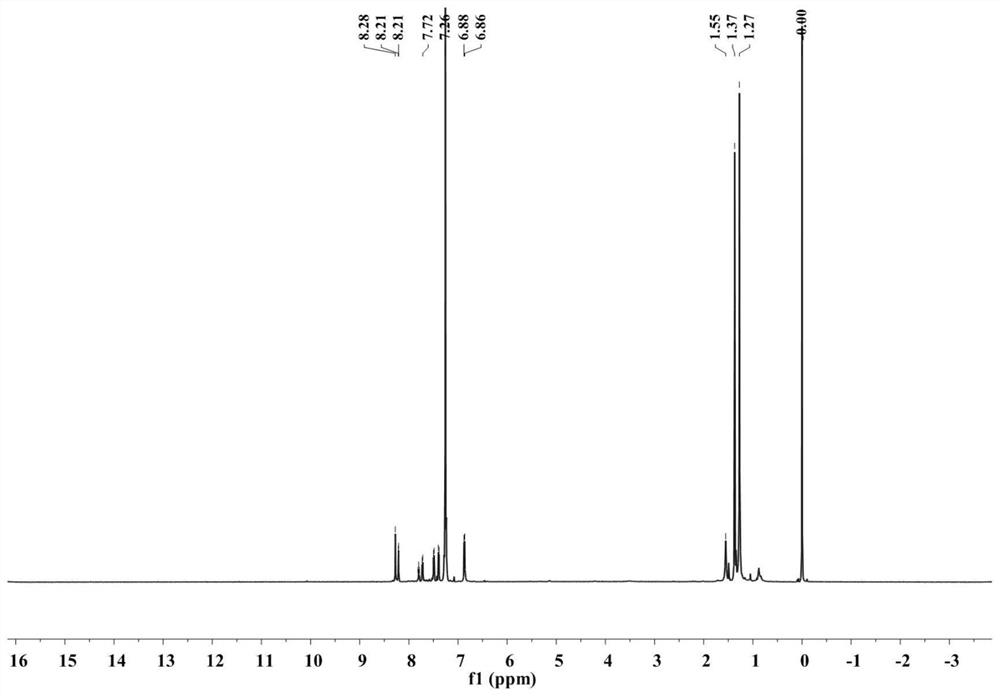
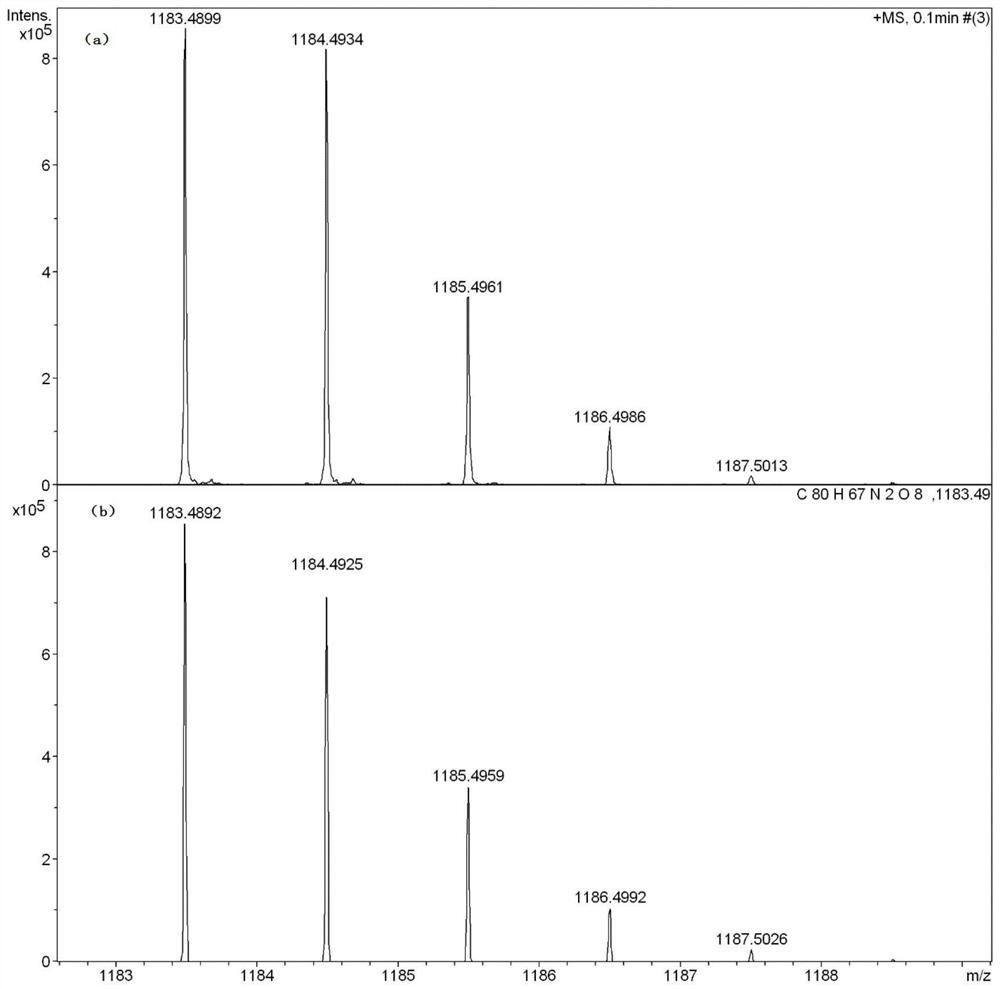
[0100] Synthesis of Gold (Ⅲ) Complex-Perylene Diimide Derivatives (In this Example, n=4)
[0101] Among them, p-bromoaniline and 2,6-di(tert-butyl)phenylpyridine are selected as raw materials.
[0102] 1) Synthesis of compound 1-1
[0103]Weigh 1.43g of 2,6-bis(tert-butyl)phenylpyridine and 2.65g of mercuric acetate into a 100mL two-necked bottle, add 40mL of ethanol and reflux at 85°C for 24 hours, then add lithium chloride-methanol solution (0.60g Lithium chloride was dissolved in 10mL of methanol), reacted at 60°C for half an hour, then added 40mL of deionized water to precipitate, filtered, washed with deionized water, and the obtained white solid a was vacuum-dried at 50°C for later use; weighed 308mg of white solid a and 246mg of potassium chloroaurate in a 100mL two-neck bottle. Under nitrogen protection, 45 mL of acetonitrile was added to the reaction system, heated to 80° C., stirred for 24 hours, cooled to room temperature, and spin-dried. A yellow solid compound ...
Embodiment 2
[0122] Synthesis of Gold (Ⅲ) Complex-Perylene Diimide Derivatives (In this Example, n=0)
[0123] Among them, p-bromoaniline and 2,6-diphenylpyridine are selected as raw materials.
[0124] 1) Synthesis of compound 1-2
[0125] Weigh 1.60g of 2,6-diphenylpyridine and 4.4g of mercuric acetate into a 100mL two-necked bottle, add 40mL of ethanol and reflux at 85°C for 24 hours, then add lithium chloride-methanol solution (1.0g of lithium chloride dissolved in 20mL of methanol), react at 60°C for half an hour, add 60mL of deionized water to precipitate precipitate, filter and wash with deionized water, the obtained white solid a is vacuum-dried at 50°C for later use; weigh 308mg of white solid a and 246mg of gold chloride Potassium acid in a 100mL two-neck bottle. Under nitrogen protection, 45 mL of acetonitrile was added to the reaction system, heated to 80° C., stirred for 24 hours, cooled to room temperature, and spin-dried. Using dichloromethane:petroleum ether (1:1) as the...
Embodiment 3
[0143] Synthesis of Gold (Ⅲ) Complex-Perylene Diimide Derivatives (In this Example, n=4)
[0144] Among them, m-bromoaniline and 2,6-di(tert-butyl)phenylpyridine are selected as raw materials.
[0145] 1) Synthesis of compound 1-1
[0146] Weigh 1.43g of 2,6-bis(tert-butyl)phenylpyridine and 2.65g of mercuric acetate into a 100mL two-necked bottle, add 40mL of ethanol and reflux at 85°C for 24 hours, then add lithium chloride-methanol solution (0.60g Lithium chloride was dissolved in 10mL of methanol), reacted at 60°C for half an hour, then added 40mL of deionized water to precipitate, filtered, washed with deionized water, and the obtained white solid a was vacuum-dried at 50°C for later use; weighed 308mg of white solid a and 246mg of potassium chloroaurate in a 100mL two-neck bottle. Under nitrogen protection, 45 mL of acetonitrile was added to the reaction system, heated to 80° C., stirred for 24 hours, cooled to room temperature, and spin-dried. A yellow solid was obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com