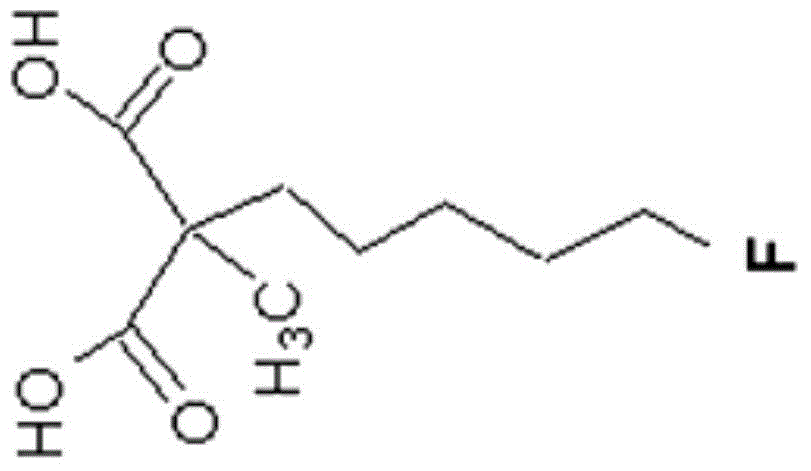
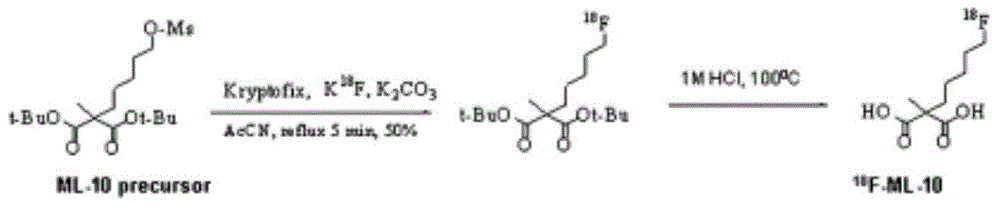
Precursors of novel PET (polyethylene terephthalate) precursor-fluoride standard ML-10 and preparation method thereof
A substance, a technology of diethyl methylmalonate, applied in the field of ML intermediates and their preparation, can solve problems such as restricting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
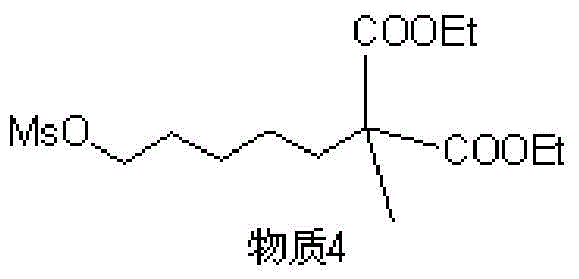
[0042] Specific embodiment 1, the structure and synthesis of material 4 and material 5, such as image 3 , Figure 4 , Figure 5 , Figure 6 shown.
[0043] Synthesis of Substance 1:
[0044] Under nitrogen protection, add 4g PPTS (pyridinium p-toluenesulfonate 16mmol, 0.2eq), 13.36g 5-bromo-1-pentanol (80mmol) and 200ml dichloromethane into a 500ml three-neck flask, dropwise add 10.08g3,4 - A solution of dihydropyran (120 mmol, 1.5 eq) in 200 ml of dichloromethane. After dropping, react overnight at room temperature. Stop the reaction, wash with water, dry the organic phase with anhydrous magnesium sulfate, filter to obtain the crude product m=25g, and separate the crude product through a 300-400 mesh silica gel column (ethyl acetate:n-hexane=5:1-2:1). The pure product m=13.7g was obtained, and the yield was 68%.
[0045] LC-MS[M+1] + =251 (100%), 253 (97%)
[0046] 1 HNMR (300MHz, CDCl 3):δ=4.55-4.60 (m, 1H), 3.81-3.92 (m, 1H), 3.69-3.80 (dt, 1H, J=6.4Hz, 9.7Hz), ...
specific Embodiment 2
[0081] Synthesis of Substance 1:
[0082] Under nitrogen protection, add 3-5g PPTS (pyridinium p-toluenesulfonate), 12-16g 5-bromo-1-pentanol and 200ml dichloromethane into a 500ml three-necked flask, drop 10-16g 3,4-dihydropyridine A solution of 200ml dichloromethane. After dropping, react overnight at room temperature. Stop the reaction, wash with water, dry the organic phase with anhydrous magnesium sulfate, filter to obtain the crude product, and separate the crude product through a 300-400 mesh silica gel column (ethyl acetate:n-hexane=5:1-2:1). Obtain pure product, productive rate 68%.
[0083] Table 1. Material 1 Synthesis Feeding Example
[0084] serial number
PPTS
5-Bromo-1-pentanol
3,4 dihydropyran
product
Yield
1
3g
12g
200ml
10g
13.7
68%
2
4g
14g
200ml
14g
15.8
68%
[0085] 3
5g
16g
200ml
16g
18.3
68%
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com