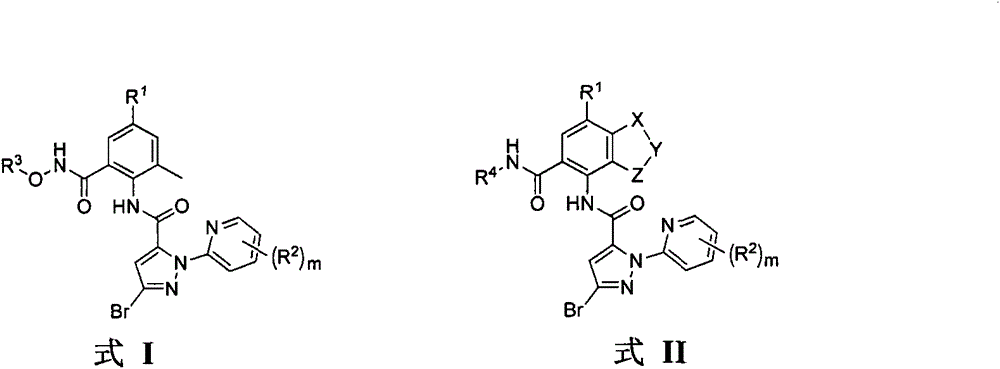
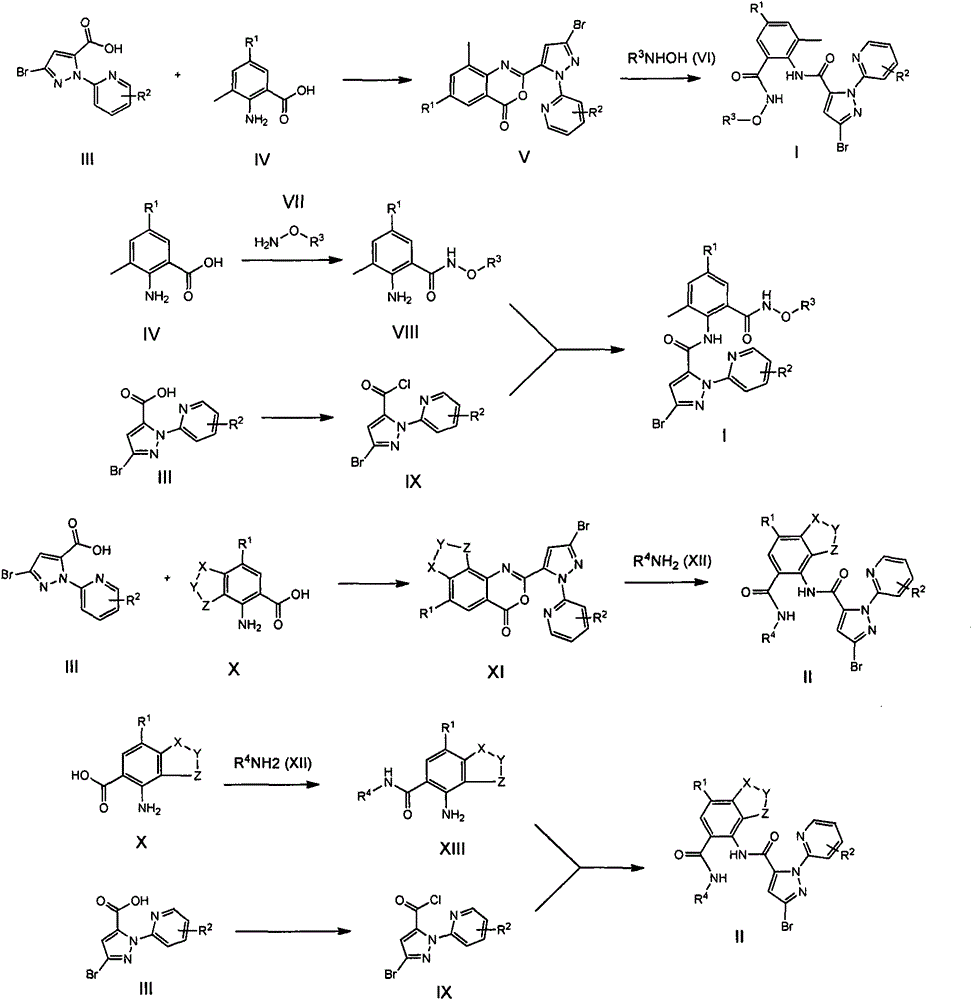
O-formammidotiazol-benzamide derivatives based on ryanodine receptor, and preparation method and applications thereof
A technology of o-formamidobenzamide and derivatives, applied in the field of o-formamidobenzamide derivatives, can solve problems such as the limited number of pesticide varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: 3-Bromo-1-(3-chloropyridin-2-yl)-N-(4-cyano-2-(methoxycarbamoyl)-6-methylphenyl)-1H- Preparation of pyrazole-5-carboxamide (Compound I-10)
[0059]
[0060] Compound I-10 can be prepared by the following method, and the specific steps are as follows:
[0061]
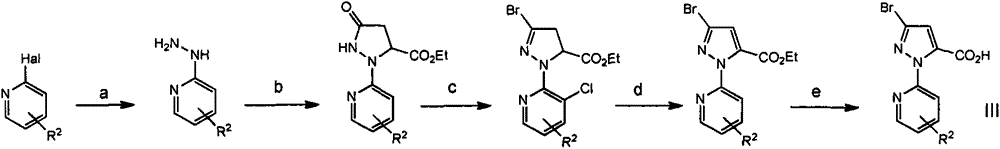
[0062] Preparation of intermediate 3-bromo-1-(3-chloro-pyridyl)-1H-pyrazole-5-carboxylic acid III-1
[0063]
[0064] Preparation of 2-(3-bromo-pyrazol-1-yl)-3-chloropyridine:
[0065] 3-Bromo-1H-pyrazole was dissolved in DMF (80 mL), 2,3-dichloro-pyridine (13.8 g) and potassium carbonate (57.3 g) were added, and the mixture was stirred at 100°C for 8 hours. After adding water, the mixture was extracted twice with ethylamine, and the combined organic layer was washed twice with water and brine, and then washed with Na 2 SO 4 Dry and concentrate in vacuum. The residue was purified by column chromatography (propylamine / ethylamine 6:1) to obtain 7 g of the title compound 2-(3-bromo-pyrazol-1-yl)-3-chloropyridin...
Embodiment 2
[0079] Example 2: 3-Bromo-N-(4-chloro-2-methyl-6-(carboxycarbamoyl)phenyl)-1-(3,5-dichloropyridin-2-yl)-1H- Preparation of pyrazole-5-carboxamide (Compound I-2)
[0080]
[0081] Compound I-2 can be prepared by the following method, and the specific steps are as follows:
[0082] Preparation of 2-(3-bromo-1H-pyrazol-1-yl)-3,5-dichloropyridine:
[0083]
[0084] This compound is carried out in the same way as the preparation of 2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine, which is prepared from 3-bromo-1H-pyrazole and 2,3,5-trichloropyridine To prepare.
[0085] Preparation of 3-bromo-1-(3,5-dichloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid (III-2):
[0086]
[0087] Compound III-2 was prepared from 2-(3-bromo-1H-pyrazol-1-yl)-3,5-dichloropyridine and carbon dioxide using the same method as preparation III-1.
[0088] Preparation of 2-amino-5-chloro-3-methylbenzoic acid (Compound IV-2):
[0089] Use the same method for preparing 2-amino-5-iodo-3-methylbenzoic acid, in which N-chlorosu...
Embodiment 3
[0096] Example 3: 3-Bromo-1-(3-chloropyridin-2-yl)-N-(4-cyano-2-(hydroxycarbamoyl)-6-methylphenyl)-1H-pyrazole Preparation of -5-carboxamide (Compound I-9):
[0097]
[0098] Compound I-9 was prepared from Intermediate V-1) through the above route using the same steps as Preparation Example 1, and the reagent compound VI) was prepared with NH 2 OH·HCl instead of CH 3 ONH 2 ·HCl.
[0099] To 2-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-4-oxo-4H-benzo[d][1 , 3] Oxazine-6-carbonitrile (V-1) (50 mg, 0.11 mmol) dissolved in CH 3 Add NH to the solution in CN (5mL) 2 OH·HCl (159.8 mg, 2.3 mmol) and Et 3 N (319.3uL, 2.3mmol), stirred at room temperature for 1 hour. The reaction mixture was filtered, and the filtrate was concentrated in vacuo and subjected to flash chromatography (dichloromethane: methanol = 10:1) to obtain a colorless solid (15 mg, yield: 28%); 1 H NMR(DMSO-d 6 , 300MHz), δ: 2.14 (s, 3H), 7.36 (s, 1H), 7.61-7.65 (dd, 1H, J = 7.8 Hz, J = 4.8 Hz), 7.75 (s, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com