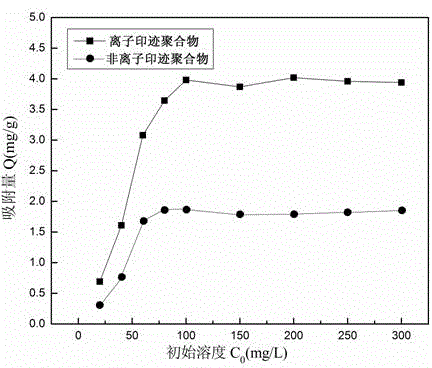
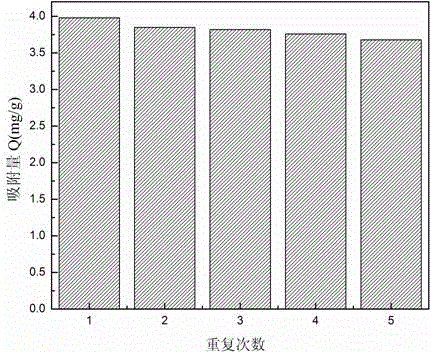
Method for preparing magnetic lithium ionic imprinting microspheres by using surface polymerization method implemented by taking macrocyclic polyethers as functional monomer
A technology of macrocyclic polyethers and functional monomers, applied in chemical instruments and methods, and other chemical processes, can solve the problems that no one has prepared lithium-ion imprinted polymers, and achieve controllable morphology, simple preparation methods, The effect of large adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Fe 3 o 4 Preparation: 13.5 g of FeCl 3 ·6H 2 O was dissolved in a 250 mL three-neck flask, nitrogen gas was introduced for 10 min, and then 6.95 g of Fe was added 2 SO 4 ·7H 2 O, continue to pass nitrogen gas for 10 min, stir rapidly, and slowly add 50 mL of concentrated ammonia water to the solution, react at 90 °C for 2 h, and dry in vacuum to obtain Fe 3 o 4 .
[0026] (2) Fe 3 o 4 SiO 2 Preparation: 5.00 g Fe 3 o 4 Add 250 mL of isopropanol and 20 mL of high-purity water to the mixed solution, stir evenly and sonicate for 15 min, then add 20 mL of ammonia and 33.3 mL of ethyl orthosilicate in sequence. After stirring and reacting at room temperature for 12 h, the product was separated by a magnet and washed with ultrapure water to neutrality to obtain Fe 3 o 4 SiO 2 .
[0027] (3) MH-Fe 3 o 4 SiO 2 Preparation: Take 5.00 g Fe 3 o 4 SiO 2 The suspension was sonicated for 20 min, then stirred rapidly to make the Fe 3 o 4 SiO 2...
Embodiment 2
[0032] (1) Fe 3 o 4 Preparation: 13.5 g of FeCl 3 ·6H 2 O was dissolved in a 250 mL three-neck flask, nitrogen gas was introduced for 5 min, and then 6.95 g of Fe was added 2 SO 4 ·7H 2 O, continue to pass nitrogen gas for 20 min, stir rapidly, and slowly add 50 mL of concentrated ammonia water to the solution, react at 90 °C for 4 h, and dry in vacuum to obtain Fe 3 o 4 .
[0033] (2) Fe 3 o 4 SiO 2 Preparation: 5.00 g Fe 3 o 4 Add 250 mL of isopropanol and 20 mL of high-purity water to the mixed solution, stir evenly and sonicate for 15 min, then add 20 mL of ammonia and 33.3 mL of ethyl orthosilicate in sequence. After stirring and reacting at room temperature for 12 h, the product was separated by a magnet and washed with ultrapure water to neutrality to obtain Fe 3 o 4 SiO 2 .
[0034] (3) MH-Fe 3 o 4 SiO 2 Preparation: Take 5.00 g Fe 3 o 4 SiO 2 The suspension was sonicated for 20 min, then stirred rapidly to make the Fe 3 o 4 SiO 2 ...
Embodiment 3
[0039] (1) Fe 3 o 4 Preparation: 13.5 g of FeCl 3 ·6H 2 O was dissolved in a 250 mL three-neck flask, nitrogen gas was introduced for 8 min, and then 6.95 g of Fe was added 2 SO 4 ·7H 2 O, continue to pass nitrogen gas for 15 min, stir rapidly, and slowly add 50 mL of concentrated ammonia water to the solution, at 90 ℃ Under the conditions of reaction for 2 h, vacuum-dried to obtain Fe 3 o 4 .
[0040] (2) Fe 3 o 4 SiO 2 Preparation: 5.00 g Fe 3 o 4 Add 250 mL of isopropanol and 20 mL of high-purity water to the mixed solution, stir evenly and sonicate for 15 min, then add 20 mL of ammonia and 33.3 mL of ethyl orthosilicate in sequence. After stirring and reacting at room temperature for 12 h, the product was separated by a magnet and washed with ultrapure water to neutrality to obtain Fe 3 o 4 SiO 2 .
[0041] (3) MH-Fe 3 o 4 SiO 2 Preparation: Take 5.00 g Fe 3 o 4 SiO 2 The suspension was sonicated for 20 min, then stirred rapidly to make ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com